User:Alar222/sandbox
 | |
| Clinical data | |
|---|---|
| Trade names | Hydroxyamfetamine, Paredrine |
| Routes of administration | Topical (ocular) |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C9H13NO |
| Molar mass | 151.206 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
4-Hydroxyamphetamine (4HA), also known as hydroxyamfetamine (INN and BAN), hydroxyamphetamine (USAN), oxamphetamine, norpholedrine, para-hydroxyamphetamine, and α-methyltyramine, is a sympathomimetic drug, that is, a drug that stimulates the sympathetic nervous system.
When used in eye drops, it dilates the pupil. 4-Hydroxyamphetamine is sold in combination with tropicamide under the brand name Paremyd.
Pharmacology
[edit]Pharmacokinetics
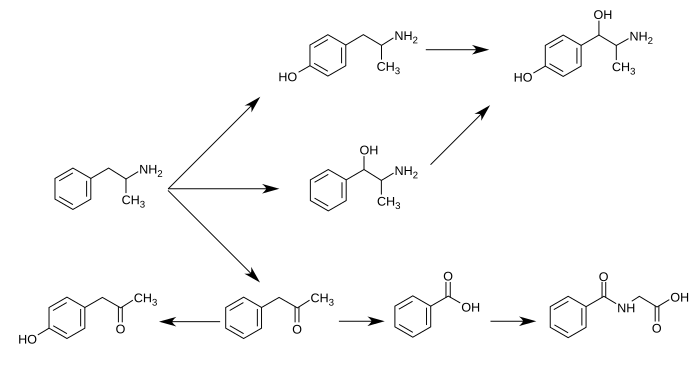
[edit]4-Hydroxyamphetamine is a major metabolite of amphetamine. In humans, amphetamine is metabolized to 4-hydroxyamphetamine by CYP2D6, which is a member of the cytochrome P450 superfamily and is found in the liver.[1][2] 4-Hydroxyamphetamine is then eliminated in the urine.[3]
Metabolic pathways of amphetamine in humans[sources 1]
|
Pharmacodynamics
[edit]4-Hydroxyamphetamine acts as an indirect sympathomimetic.[15] When injected into the bloodstream or into the brain, it disrupts the regulation of serotonin (5-hydroxytryptamine or 5-HT) by inhibiting the activity of a family of enzymes called monoamine oxidases (MAOs), particularly type A (MAO-A). The inhibition of MAO-A prevents metabolism of serotonin in the presynaptic terminal, and thus causes a build up of serotonin. Excessive amounts of serotonin are then released into the synaptic cleft. The released serotonin binds to serotonin receptors on the postsynaptic terminal, causing excessive nerve response. Reuptake of unbound serotonin perpetuates the response by replenishing the any deficit of serotonin in the presynaptic terminal that might have been caused by the release of serotonin into the synaptic cleft.[16]
4-Hydroxyamphetamine is also an agonist of human TAAR1.[17]
Diagnostic Use
[edit]If instilled in the eye using an appropriate eye drop dosage and formulation, 4-hydroxyamphetamine causes the release of norepinephrine from the nerve synapses leading to mydriasis.[18] 4-Hydroxyamphetamine hydrobromide is a known formulation that can be used as a diagnostic tool for Horner's syndrome. Patients with Horner’s syndrome exhibit anisocoria brought about by lesions on the nerves the connect to the nasociliary branch of the ophthalmic nerve.[19] Application of 4-hydroxyamphetamine to the eye can indicate whether the lesion is preganglionic or postganglionic based on the pupil’s response. If the pupil dilates, the lesion is preganglionic. If the pupil does not dilate, the lesion is postganglionic.[20]
Unfortunately, 4-hydroxyamphetamine has a few limitations to its use as a diagnostic tool. If it is intended as an immediate follow up to another mydriatic drug (cocaine or apraclonidine), then the patient must wait anywhere from a day to a week before 4-hydroxyamphetamine can be administered.[21][22] It also has the tendency to falsely localize lesions in cases of acute onset; in cases where a postganglionic lesion is present but the nerve still responds to residual norepinephrine; or in cases in which unrelated nerve damage masks the presence of a preganglionic lesion.[23][24][25]
Commercialization
[edit]Hydroxyamphetamine is a known component of two controlled (prescription only), name-brand ophthalmic mydriatics: Paredrine and Paremyd. Paredrine consists of a 1% solution of hydroxyamphetamine hydrobromide[26] while Paremyd consists of a combination of 1% hydroxyamphetamine hydrobromide and 0.25% tropicamide. [27] In the 1990s, the trade name rights, patents, and new drug applications (NDAs) for the two formulations were exchanged among a few different manufacturers after a shortage of the raw material required for their production, which caused both drugs to be indefinitely removed from the market [1]. Around 1997, Akorn, Inc., obtained the rights to both Paredrine and Paremyd[2], and in 2002, the company reintroduced Paremyd to the market as a fast acting ophthalmic mydriatic and cycloplegic[3][4][5].
See also
[edit]External links
[edit]- p-Hydroxyamphetamine at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
Category:Phenols Category:Sympathomimetics Category:Amphetamine Category:Amphetamines Category:TAAR1 agonists Category:Norepinephrine-dopamine releasing agents
References
[edit]- ^ Markowitz JS & Patrick KS (2001). "Pharmacokinetic and pharmacodynamic drug interactions in the treatment of attention-deficit hyperactivity disorder." Clinical pharmacokinetics. 40(10):757-758 PMID 11707061
- ^ Haefely W, Bartholini G, & Pletscher A (1976). "Monoaminergic drugs: general pharmacology." Pharmacology & therapeutics. Part B: General & systematic pharmacology. 2(1):197 PMID 817330
- ^ Cho AK & Wright J (1978). "Pathways of metabolism of amphetamine and related compounds." Life sciences. 22(5):368 PMID 347211
- ^ "Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. Shire US Inc. December 2013. pp. 12–13. Retrieved December 30, 2013.
- ^ a b Glennon RA (2013). "Phenylisopropylamine stimulants: amphetamine-related agents". In Lemke TL, Williams DA, Roche VF, Zito W (eds.). Foye's principles of medicinal chemistry (7th ed.). Philadelphia, US: Wolters Kluwer Health/Lippincott Williams & Wilkins. pp. 646–648. ISBN 9781609133450.
The simplest unsubstituted phenylisopropylamine, 1-phenyl-2-aminopropane, or amphetamine, serves as a common structural template for hallucinogens and psychostimulants. Amphetamine produces central stimulant, anorectic, and sympathomimetic actions, and it is the prototype member of this class (39). ... The phase 1 metabolism of amphetamine analogs is catalyzed by two systems: cytochrome P450 and flavin monooxygenase. ... Amphetamine can also undergo aromatic hydroxylation to p-hydroxyamphetamine. ... Subsequent oxidation at the benzylic position by DA β-hydroxylase affords p-hydroxynorephedrine. Alternatively, direct oxidation of amphetamine by DA β-hydroxylase can afford norephedrine.
- ^ Taylor KB (January 1974). "Dopamine-beta-hydroxylase. Stereochemical course of the reaction" (PDF). Journal of Biological Chemistry. 249 (2): 454–458. doi:10.1016/S0021-9258(19)43051-2. PMID 4809526. Retrieved November 6, 2014.
Dopamine-β-hydroxylase catalyzed the removal of the pro-R hydrogen atom and the production of 1-norephedrine, (2S,1R)-2-amino-1-hydroxyl-1-phenylpropane, from d-amphetamine.
- ^ Krueger SK, Williams DE (June 2005). "Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism". Pharmacology & Therapeutics. 106 (3): 357–387. doi:10.1016/j.pharmthera.2005.01.001. PMC 1828602. PMID 15922018.
Table 5: N-containing drugs and xenobiotics oxygenated by FMO - ^ Cashman JR, Xiong YN, Xu L, Janowsky A (March 1999). "N-oxygenation of amphetamine and methamphetamine by the human flavin-containing monooxygenase (form 3): role in bioactivation and detoxication". Journal of Pharmacology and Experimental Therapeutics. 288 (3): 1251–1260. PMID 10027866.
- ^ Santagati NA, Ferrara G, Marrazzo A, Ronsisvalle G (September 2002). "Simultaneous determination of amphetamine and one of its metabolites by HPLC with electrochemical detection". Journal of Pharmaceutical and Biomedical Analysis. 30 (2): 247–255. doi:10.1016/S0731-7085(02)00330-8. PMID 12191709.
- ^ a b c Sjoerdsma A, von Studnitz W (April 1963). "Dopamine-beta-oxidase activity in man, using hydroxyamphetamine as substrate". British Journal of Pharmacology and Chemotherapy. 20 (2): 278–284. doi:10.1111/j.1476-5381.1963.tb01467.x. PMC 1703637. PMID 13977820.
Hydroxyamphetamine was administered orally to five human subjects ... Since conversion of hydroxyamphetamine to hydroxynorephedrine occurs in vitro by the action of dopamine-β-oxidase, a simple method is suggested for measuring the activity of this enzyme and the effect of its inhibitors in man. ... The lack of effect of administration of neomycin to one patient indicates that the hydroxylation occurs in body tissues. ... a major portion of the β-hydroxylation of hydroxyamphetamine occurs in non-adrenal tissue. Unfortunately, at the present time one cannot be completely certain that the hydroxylation of hydroxyamphetamine in vivo is accomplished by the same enzyme which converts dopamine to noradrenaline.
- ^ Badenhorst CP, van der Sluis R, Erasmus E, van Dijk AA (September 2013). "Glycine conjugation: importance in metabolism, the role of glycine N-acyltransferase, and factors that influence interindividual variation". Expert Opinion on Drug Metabolism & Toxicology. 9 (9): 1139–1153. doi:10.1517/17425255.2013.796929. PMID 23650932. S2CID 23738007.
Figure 1. Glycine conjugation of benzoic acid. The glycine conjugation pathway consists of two steps. First benzoate is ligated to CoASH to form the high-energy benzoyl-CoA thioester. This reaction is catalyzed by the HXM-A and HXM-B medium-chain acid:CoA ligases and requires energy in the form of ATP. ... The benzoyl-CoA is then conjugated to glycine by GLYAT to form hippuric acid, releasing CoASH. In addition to the factors listed in the boxes, the levels of ATP, CoASH, and glycine may influence the overall rate of the glycine conjugation pathway.
- ^ Horwitz D, Alexander RW, Lovenberg W, Keiser HR (May 1973). "Human serum dopamine-β-hydroxylase. Relationship to hypertension and sympathetic activity". Circulation Research. 32 (5): 594–599. doi:10.1161/01.RES.32.5.594. PMID 4713201. S2CID 28641000.
The biologic significance of the different levels of serum DβH activity was studied in two ways. First, in vivo ability to β-hydroxylate the synthetic substrate hydroxyamphetamine was compared in two subjects with low serum DβH activity and two subjects with average activity. ... In one study, hydroxyamphetamine (Paredrine), a synthetic substrate for DβH, was administered to subjects with either low or average levels of serum DβH activity. The percent of the drug hydroxylated to hydroxynorephedrine was comparable in all subjects (6.5-9.62) (Table 3).
- ^ Freeman JJ, Sulser F (December 1974). "Formation of p-hydroxynorephedrine in brain following intraventricular administration of p-hydroxyamphetamine". Neuropharmacology. 13 (12): 1187–1190. doi:10.1016/0028-3908(74)90069-0. PMID 4457764.
In species where aromatic hydroxylation of amphetamine is the major metabolic pathway, p-hydroxyamphetamine (POH) and p-hydroxynorephedrine (PHN) may contribute to the pharmacological profile of the parent drug. ... The location of the p-hydroxylation and β-hydroxylation reactions is important in species where aromatic hydroxylation of amphetamine is the predominant pathway of metabolism. Following systemic administration of amphetamine to rats, POH has been found in urine and in plasma.
The observed lack of a significant accumulation of PHN in brain following the intraventricular administration of (+)-amphetamine and the formation of appreciable amounts of PHN from (+)-POH in brain tissue in vivo supports the view that the aromatic hydroxylation of amphetamine following its systemic administration occurs predominantly in the periphery, and that POH is then transported through the blood-brain barrier, taken up by noradrenergic neurones in brain where (+)-POH is converted in the storage vesicles by dopamine β-hydroxylase to PHN. - ^ Matsuda LA, Hanson GR, Gibb JW (December 1989). "Neurochemical effects of amphetamine metabolites on central dopaminergic and serotonergic systems". Journal of Pharmacology and Experimental Therapeutics. 251 (3): 901–908. PMID 2600821.
The metabolism of p-OHA to p-OHNor is well documented and dopamine-β hydroxylase present in noradrenergic neurons could easily convert p-OHA to p-OHNor after intraventricular administration.
- ^ Cho AK & Wright J (1978). "Pathways of metabolism of amphetamine and related compounds." Life sciences. 22(5):368 PMID 347211
- ^ Nakagawasai O, et al. (2004). "Monoamine oxidase and head-twitch response in mice. Mechanisms of alpha-methylated substrate derivatives." Neurotoxicology. 25(1-2):223-232 PMID 14697897
- ^ "Articleid 50034244". Binding Database. Retrieved April 29, 2014.
- ^ Lepore FE (1985). "Diagnostic pharmacology of the pupil." Clinical neuropharmacology. 8(1):31 PMID 3884149
- ^ Walton KA & Buono LM (2003). "Horner syndrome." Current opinion in ophthalmology. 14(6):357 PMID 14615640
- ^ Walton KA & Buono LM (2003). "Horner syndrome." Current opinion in ophthalmology. 14(6):359 PMID 14615640
- ^ Davagnanam I, Fraser CL, Miszkiel K, Daniel CS, & Plant GT (2013). "Adult Horner's syndrome: a combined clinical, pharmacological, and imaging algorithm." Eye (London, England). 27(3):291,294 PMID 23370415
- ^ Lepore FE (1985). "Diagnostic pharmacology of the pupil." Clinical neuropharmacology. 8(1):29 PMID 3884149
- ^ Davagnanam I, Fraser CL, Miszkiel K, Daniel CS, & Plant GT (2013). "Adult Horner's syndrome: a combined clinical, pharmacological, and imaging algorithm." Eye (London, England). 27(3):294 PMID 23370415
- ^ Walton KA & Buono LM (2003). "Horner syndrome." Current opinion in ophthalmology. 14(6):359 PMID 14615640
- ^ Lepore FE (1985). "Diagnostic pharmacology of the pupil." Clinical neuropharmacology. 8(1):31-32 PMID 3884149
- ^ Slamovits TL and JS Glase (1999). 'The Pupils and Accommodation." in Neuro-ophthamology, ed Glaser JS (Lippincott, Williams, & Wilkins; Philadelphia, PA), p 543 Google Books Accessed 10 Dec 2014
- ^ FDA Orange Book. Search Proprietary Name: Paremyd. Accessed 03 Dec 2014
Cite error: There are <ref group=sources> tags on this page, but the references will not show without a {{reflist|group=sources}} template (see the help page).
Cite error: There are <ref group=note> tags on this page, but the references will not show without a {{reflist|group=note}} template (see the help page).