Tellurous acid
Appearance
(Redirected from Tellurium acid)
| |
| Names | |
|---|---|
| IUPAC name
Tellurous acid
| |
| Other names
Tellurium dioxide hydrate, tellurium(IV) oxide hydrate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.030.145 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
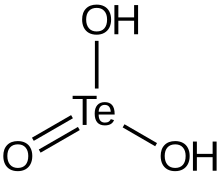
| H2TeO3 | |
| Molar mass | 177.616 grams |
| Appearance | colorless crystals |
| Density | ~ 3 g/cm3 |
| Boiling point | decomposes |
| negligible | |
| Acidity (pKa) | pKa1 = 2.48, pKa2 = 7.70 [1] |
| Conjugate base | Tellurite |
| Structure | |
| unknown | |
| pyramidal at Te | |
| Related compounds | |
Other anions
|
Selenous acid Sulfurous acid |
Other cations
|
Sodium tellurite |
Related compounds
|
Telluric acid Selenic acid Sulfuric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tellurous acid is an inorganic compound with the formula H2TeO3. It is the oxoacid of tellurium(IV).[2] This compound is not well characterized. An alternative way of writing its formula is (HO)2TeO. In principle, tellurous acid would form by treatment of tellurium dioxide with water, that is by hydrolysis. The related conjugate base is well known in the form of several salts such as potassium hydrogen tellurite, KHTeO3.
Properties
[edit]In contrast to the analogous compound selenous acid, tellurous acid is only metastable. Most tellurite salts contain the TeO2−
3 ion. Oxidation of its aqueous solution with hydrogen peroxide gives the tellurate ion. It is usually prepared as an aqueous solution where it acts as a weak acid.[1][3]
- H2TeO3 + H2O ⇌ H3O+ + HTeO−
3 Ka1 = 2×10−3 - HTeO−
3 + H2O ⇌ H3O+ + TeO2−
3 Ka2 = 1×10−8
References
[edit]- ^ a b Catherine E. Housecroft; Alan G. Sharpe (2008). "Chapter 16: The group 16 elements". Inorganic Chemistry (3rd ed.). Pearson. p. 524. ISBN 978-0-13-175553-6.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ "Ionization Constants for Weak Acids". www.austincc.edu. Archived from the original on 2014-10-20.
