Garnet
| Garnet | |
|---|---|
 | |
| General | |
| Category | Nesosilicate |
| Formula (repeating unit) | The general formula X3Y2(SiO4)3 |
| IMA symbol | Grt[1] |
| Crystal system | Isometric |
| Crystal class | |
| Space group | Ia3d |
| Identification | |
| Color | virtually all colors, blue is rare |
| Crystal habit | Rhombic dodecahedron or cubic |
| Cleavage | Indistinct |
| Fracture | conchoidal to uneven |
| Mohs scale hardness | 6.5–7.5 |
| Luster | vitreous to resinous |
| Streak | White |
| Diaphaneity | Can form with any diaphaneity, translucent is common |
| Specific gravity | 3.1–4.3 |
| Polish luster | vitreous to subadamantine[2] |
| Optical properties | Single refractive, often anomalous double refractive[2] |
| Refractive index | 1.72–1.94 |
| Birefringence | None |
| Pleochroism | None |
| Ultraviolet fluorescence | variable |
| Other characteristics | variable magnetic attraction |
| Major varieties | |
| Pyrope | Mg3Al2Si3O12 |
| Almandine | Fe3Al2Si3O12 |
| Spessartine | Mn3Al2Si3O12 |
| Andradite | Ca3Fe2Si3O12 |
| Grossular | Ca3Al2Si3O12 |
| Uvarovite | Ca3Cr2Si3O12 |
Garnets ( /ˈɡɑːrnɪt/) are a group of silicate minerals that have been used since the Bronze Age as gemstones and abrasives.
All species of garnets possess similar physical properties and crystal forms, but differ in chemical composition. The different species are pyrope, almandine, spessartine, grossular (varieties of which are hessonite or cinnamon-stone and tsavorite), uvarovite and andradite. The garnets make up two solid solution series: pyrope-almandine-spessartine (pyralspite), with the composition range [Mg,Fe,Mn]3Al2(SiO4)3; and uvarovite-grossular-andradite (ugrandite), with the composition range Ca3[Cr,Al,Fe]2(SiO4)3.
Etymology
[edit]The word garnet comes from the 14th-century Middle English word gernet, meaning 'dark red'. It is borrowed from Old French grenate from Latin granatus, from granum ('grain, seed').[3] This is possibly a reference to mela granatum or even pomum granatum ('pomegranate',[4] Punica granatum), a plant whose fruits contain abundant and vivid red seed covers (arils), which are similar in shape, size, and color to some garnet crystals.[5] Hessonite garnet is also named 'gomed' in Indian literature and is one of the 9 jewels in Vedic astrology that compose the Navaratna.[6]
Physical properties
[edit]Properties
[edit]
Garnet species are found in every colour, with reddish shades most common. Blue garnets are the rarest and were first reported in the 1990s.[7][8][9][10]
Garnet species' light transmission properties can range from the gemstone-quality transparent specimens to the opaque varieties used for industrial purposes as abrasives. The mineral's lustre is categorized as vitreous (glass-like) or resinous (amber-like).[3]
Crystal structure
[edit]Garnets are nesosilicates having the general formula X3Y2(SiO
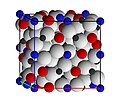
4)3. The X site is usually occupied by divalent cations (Ca, Mg, Fe, Mn)2+ and the Y site by trivalent cations (Al3+, Fe3+, Cr3+) in an octahedral/tetrahedral framework with [SiO4]4− occupying the tetrahedra.[11] Garnets are most often found in the dodecahedral crystal habit, but are also commonly found in the trapezohedron habit as well as the hexoctahedral habit.[3] They crystallize in the cubic system, having three axes that are all of equal length and perpendicular to each other, but are never actually cubic because, despite being isometric, the {100} and {111} families of planes are depleted.[3] Garnets do not have any cleavage planes, so when they fracture under stress, sharp, irregular (conchoidal) pieces are formed.[12]
-
Crystal structure of pyrope garnet. White spheres are oxygen; black, silicon; blue, aluminium; and red, magnesium.
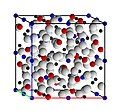
-
Same view, with ion sizes reduced to better show all ions
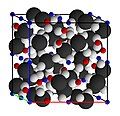
-
Silicon ion size exaggerated to emphasize silica tetrahedra
Hardness
[edit]Because the chemical composition of garnet varies, the atomic bonds in some species are stronger than in others. As a result, this mineral group shows a range of hardness on the Mohs scale of about 6.0 to 7.5.[13] The harder species like almandine are often used for abrasive purposes.[14]
Magnetics used in garnet series identification
[edit]For gem identification purposes, a pick-up response to a strong neodymium magnet separates garnet from all other natural transparent gemstones commonly used in the jewelry trade. Magnetic susceptibility measurements in conjunction with refractive index can be used to distinguish garnet species and varieties, and determine the composition of garnets in terms of percentages of end-member species within an individual gem.[15]
Garnet group end member species
[edit]Pyralspite garnets – aluminium in Y site
[edit]- Almandine: Fe3Al2(SiO4)3
- Pyrope: Mg3Al2(SiO4)3
- Spessartine: Mn3Al2(SiO4)3
Almandine
[edit]
Almandine, sometimes incorrectly called almandite, is the modern gem known as carbuncle (though originally almost any red gemstone was known by this name).[16] The term "carbuncle" is derived from the Latin meaning "live coal" or burning charcoal. The name Almandine is a corruption of Alabanda, a region in Asia Minor where these stones were cut in ancient times. Chemically, almandine is an iron-aluminium garnet with the formula Fe3Al2(SiO4)3; the deep red transparent stones are often called precious garnet and are used as gemstones (being the most common of the gem garnets).[17] Almandine occurs in metamorphic rocks like mica schists, associated with minerals such as staurolite, kyanite, andalusite, and others.[18] Almandine has nicknames of Oriental garnet,[19] almandine ruby, and carbuncle.[16]
Pyrope
[edit]Pyrope (from the Greek pyrōpós meaning "firelike")[3] is red in color and chemically an aluminium silicate with the formula Mg3Al2(SiO4)3, though the magnesium can be replaced in part by calcium and ferrous iron. The color of pyrope varies from deep red to black. Pyrope and spessartine gemstones have been recovered from the Sloan diamondiferous kimberlites in Colorado, from the Bishop Conglomerate and in a Tertiary age lamprophyre at Cedar Mountain in Wyoming.[20]
A variety of pyrope from Macon County, North Carolina is a violet-red shade and has been called rhodolite, Greek for "rose". In chemical composition it may be considered as essentially an isomorphous mixture of pyrope and almandine, in the proportion of two parts pyrope to one part almandine.[21] Pyrope has tradenames some of which are misnomers; Cape ruby, Arizona ruby, California ruby, Rocky Mountain ruby, and Bohemian ruby from the Czech Republic.[16]
Pyrope is an indicator mineral for high-pressure rocks. Mantle-derived rocks (peridotites and eclogites) commonly contain a pyrope variety.[22]
Spessartine
[edit]
Spessartine or spessartite is manganese aluminium garnet, Mn3Al2(SiO4)3. Its name is derived from Spessart in Bavaria.[3] It occurs most often in skarns,[3] granite pegmatite and allied rock types,[23] and in certain low grade metamorphic phyllites. Spessartine of an orange-yellow is found in Madagascar.[24] Violet-red spessartines are found in rhyolites in Colorado[21] and Maine.[citation needed]
Pyrope–spessartine (blue garnet or color-change garnet)
[edit]Blue pyrope–spessartine garnets were discovered in the late 1990s in Bekily, Madagascar. This type has also been found in parts of the United States, Russia, Kenya, Tanzania, and Turkey. It changes color from blue-green to purple depending on the color temperature of viewing light, as a result of the relatively high amounts of vanadium (about 1 wt.% V2O3).[9]
Other varieties of color-changing garnets exist. In daylight, their color ranges from shades of green, beige, brown, gray, and blue, but in incandescent light, they appear a reddish or purplish/pink color.[25]
This is the rarest type of garnet. Because of its color-changing quality, this kind of garnet resembles alexandrite.[26]
Ugrandite group – calcium in X site
[edit]Andradite
[edit]Andradite is a calcium-iron garnet, Ca3Fe2(SiO4)3, is of variable composition and may be red, yellow, brown, green or black.[3] The recognized varieties are demantoid (green), melanite (black),[3] and topazolite (yellow or green). The red-brown translucent variety of colophonite is recognized as a partially obsolete name.[27] Andradite is found in skarns[3] and in deep-seated igneous rocks like syenite[28] as well as serpentines[29] and greenschists.[30] Demantoid is one of the most prized of garnet varieties.[31]
Grossular
[edit]

Grossular is a calcium-aluminium garnet with the formula Ca3Al2(SiO4)3, though the calcium may in part be replaced by ferrous iron and the aluminium by ferric iron. The name grossular is derived from the botanical name for the gooseberry, grossularia, in reference to the green garnet of this composition that is found in Siberia. Other shades include cinnamon brown (cinnamon stone variety), red, and yellow.[3] Because of its inferior hardness to zircon, which the yellow crystals resemble, they have also been called hessonite from the Greek meaning inferior.[32] Grossular is found in skarns,[3] contact metamorphosed limestones with vesuvianite, diopside, wollastonite and wernerite.
Grossular garnet from Kenya and Tanzania has been called tsavorite. Tsavorite was first described in the 1960s in the Tsavo area of Kenya, from which the gem takes its name.[33][34]
Uvarovite
[edit]Uvarovite is a calcium chromium garnet with the formula Ca3Cr2(SiO4)3. This is a rather rare garnet, bright green in color, usually found as small crystals associated with chromite in peridotite, serpentinite, and kimberlites. It is found in crystalline marbles and schists in the Ural Mountains of Russia and Outokumpu, Finland. Uvarovite is named for Count Uvaro, a Russian imperial statesman.[3]
Less common species
[edit]- Calcium in X site
- Goldmanite: Ca3(V3+,Al,Fe3+)2(SiO4)3
- Kimzeyite: Ca3(Zr, Ti)2[(Si,Al,Fe3+)O4]3
- Morimotoite: Ca3Ti4+Fe2+(SiO4)3
- Schorlomite: Ca3Ti4+2(SiO4)(Fe3+O4)2
- Hydroxide bearing – calcium in X site
- Hydrogrossular: Ca3Al2(SiO4)3−x(OH)4x
- Hibschite: Ca3Al2(SiO4)3−x(OH)4x (where x is between 0.2 and 1.5)
- Katoite: Ca3Al2(SiO4)3−x(OH)4x (where x is greater than 1.5)
- Hydrogrossular: Ca3Al2(SiO4)3−x(OH)4x
- Magnesium or manganese in X site
- Knorringite: Mg3Cr2(SiO4)3
- Majorite: Mg3(Fe2+Si)(SiO4)3
- Calderite: Mn3Fe3+2(SiO4)3
Knorringite
[edit]Knorringite is a magnesium-chromium garnet species with the formula Mg3Cr2(SiO4)3. Pure endmember knorringite never occurs in nature. Pyrope rich in the knorringite component is only formed under high pressure and is often found in kimberlites. It is used as an indicator mineral in the search for diamonds.[35]
Garnet structural group
[edit]- Formula: X3Z2(TO4)3 (X = Ca, Fe, etc., Z = Al, Cr, etc., T = Si, As, V, Fe, Al)
- All are cubic or strongly pseudocubic.
| IMA/CNMNC Nickel-Strunz Mineral class |
Mineral name | Formula | Crystal system | Point group | Space group |
|---|---|---|---|---|---|
| 04 Oxide | Bitikleite-(SnAl) | Ca3SnSb(AlO4)3 | isometric | m3m | Ia3d |
| 04 Oxide | Bitikleite-(SnFe) | Ca3(SnSb5+)(Fe3+O4)3 | isometric | m3m | Ia3d |
| 04 Oxide | Bitikleite-(ZrFe) | Ca3SbZr(Fe3+O4)3 | isometric | m3m | Ia3d |
| 04 Tellurate | Yafsoanite | Ca3Zn3(Te6+O6)2 | isometric | m3m or 432 |
Ia3d or I4132 |
| 08 Arsenate | Berzeliite | NaCa2Mg2(AsO4)3 | isometric | m3m | Ia3d |
| 08 Vanadate | Palenzonaite | NaCa2Mn2+2(VO4)3 | isometric | m3m | Ia3d |
| 08 Vanadate | Schäferite | NaCa2Mg2(VO4)3 | isometric | m3m | Ia3d |
- IMA/CNMNC – Nickel-Strunz – Mineral subclass: 09.A Nesosilicate
- Nickel-Strunz classification: 09.AD.25
| Mineral name | Formula | Crystal system | Point group | Space group |
|---|---|---|---|---|
| Almandine | Fe2+3Al2(SiO4)3 | isometric | m3m | Ia3d |
| Andradite | Ca3Fe3+2(SiO4)3 | isometric | m3m | Ia3d |
| Calderite | Mn+23Fe+32(SiO4)3 | isometric | m3m | Ia3d |
| Goldmanite | Ca3V3+2(SiO4)3 | isometric | m3m | Ia3d |
| Grossular | Ca3Al2(SiO4)3 | isometric | m3m | Ia3d |
| Henritermierite | Ca3Mn3+2(SiO4)2(OH)4 | tetragonal | 4/mmm | I41/acd |
| Hibschite | Ca3Al2(SiO4)(3−x)(OH)4x (x= 0.2–1.5) | isometric | m3m | Ia3d |
| Katoite | Ca3Al2(SiO4)(3−x)(OH)4x (x= 1.5–3) | isometric | m3m | Ia3d |
| Kerimasite | Ca3Zr2(Fe+3O4)2(SiO4) | isometric | m3m | Ia3d |
| Kimzeyite | Ca3Zr2(Al+3O4)2(SiO4) | isometric | m3m | Ia3d |
| Knorringite | Mg3Cr2(SiO4)3 | isometric | m3m | Ia3d |
| Majorite | Mg3(Fe2+Si)(SiO4)3 | tetragonal | 4/m or 4/mmm |
I41/a or I41/acd |
| Menzerite-(Y) | Y2CaMg2(SiO4)3 | isometric | m3m | Ia3d |
| Momoiite | Mn2+3V3+2(SiO4)3 | isometric | m3m | Ia3d |
| Morimotoite | Ca3(Fe2+Ti4+)(SiO4)3 | isometric | m3m | Ia3d |
| Pyrope | Mg3Al2(SiO4)3 | isometric | m3m | Ia3d |
| Schorlomite | Ca3Ti4+2(Fe3+O4)2(SiO4) | isometric | m3m | Ia3d |
| Spessartine | Mn2+3Al2(SiO4)3 | isometric | m3m | Ia3d |
| Toturite | Ca3Sn2(Fe3+O4)2(SiO4) | isometric | m3m | Ia3d |
| Uvarovite | Ca3Cr2(SiO4)3 | isometric | m3m | Ia3d |
- References: Mindat.org; mineral name, chemical formula and space group (American Mineralogist Crystal Structure Database) of the IMA Database of Mineral Properties/ RRUFF Project, Univ. of Arizona, was preferred most of the time. Minor components in formulae have been left out to highlight the dominant chemical endmember that defines each species.
Synthetic garnets
[edit]Also known as rare-earth garnets.
The crystallographic structure of garnets has been expanded from the prototype to include chemicals with the general formula A3B2(CO4)3. Besides silicon, a large number of elements have been put on the C site, including germanium, gallium, aluminum, vanadium and iron.[36]
Yttrium aluminium garnet (YAG), Y3Al2(AlO4)3, is used for synthetic gemstones. Due to its fairly high refractive index, YAG was used as a diamond simulant in the 1970s until the methods of producing the more advanced simulant cubic zirconia in commercial quantities were developed. When doped with neodymium (Nd3+), erbium or gadolinium YAG may be used as the lasing medium in Nd:YAG lasers,[37] Er:YAG lasers and Gd:YAG lasers respectively. These doped YAG lasers are used in medical procedures including laser skin resurfacing, dentistry, and ophthalmology.[38][39][40]
Interesting magnetic properties arise when the appropriate elements are used. In yttrium iron garnet (YIG), Y3Fe2(FeO4)3, the five iron(III) ions occupy two octahedral and three tetrahedral sites, with the yttrium(III) ions coordinated by eight oxygen ions in an irregular cube. The iron ions in the two coordination sites exhibit different spins, resulting in magnetic behavior. YIG is a ferrimagnetic material having a Curie temperature of 550 K. Yttrium iron garnet can be made into YIG spheres, which serve as magnetically tunable filters and resonators for microwave frequencies.[41]
Lutetium aluminium garnet (LuAG), Al5Lu3O12, is an inorganic compound with a unique crystal structure primarily known for its use in high-efficiency laser devices. LuAG is also useful in the synthesis of transparent ceramics.[42] LuAG is particularly favored over other crystals for its high density and thermal conductivity; it has a relatively small lattice constant in comparison to the other rare-earth garnets, which results in a higher density producing a crystal field with narrower linewidths and greater energy level splitting in absorption and emission.[43]
Terbium gallium garnet (TGG), Tb3Ga5O12, is a Faraday rotator material with excellent transparency properties and is very resistant to laser damage. TGG can be used in optical isolators for laser systems, in optical circulators for fiber optic systems, in optical modulators, and in current and magnetic field sensors.[44]
Another example is gadolinium gallium garnet (GGG), Gd3Ga2(GaO4)3 which is synthesized for use as a substrate for liquid-phase epitaxy of magnetic garnet films for bubble memory and magneto-optical applications.[citation needed]
Geological importance
[edit]

The mineral garnet is commonly found in metamorphic and to a lesser extent, igneous rocks. Most natural garnets are compositionally zoned and contain inclusions.[45] Its crystal lattice structure is stable at high pressures and temperatures and is thus found in green-schist facies metamorphic rocks including gneiss, hornblende schist, and mica schist.[46] The composition that is stable at the pressure and temperature conditions of Earth's mantle is pyrope, which is often found in peridotites and kimberlites, as well as the serpentines that form from them.[46] Garnets are unique in that they can record the pressures and temperatures of peak metamorphism and are used as geobarometers and geothermometers in the study of geothermobarometry which determines "P-T Paths", Pressure-Temperature Paths. Garnets are used as an index mineral in the delineation of isograds in metamorphic rocks.[46] Compositional zoning and inclusions can mark the change from growth of the crystals at low temperatures to higher temperatures.[47] Garnets that are not compositionally zoned more than likely experienced ultra high temperatures (above 700 °C) that led to diffusion of major elements within the crystal lattice, effectively homogenizing the crystal[47] or they were never zoned. Garnets can also form metamorphic textures that can help interpret structural histories.[47]
In addition to being used to devolve conditions of metamorphism, garnets can be used to date certain geologic events. Garnet has been developed as a U-Pb geochronometer, to date the age of crystallization[48] as well as a thermochronometer in the (U-Th)/He system[49] to date timing of cooling below a closure temperature.
Garnets can be chemically altered and most often alter to serpentine, talc, and chlorite.[46]
Largest garnet crystal
[edit]The open-pit Barton Garnet Mine, located at Gore Mountain in the Adirondack Mountains, yields the world's largest single crystals of garnet; diameters range from 5 to 35 cm and commonly average 10–18 cm.[50]
Gore Mountain garnets are unique in many respects, and considerable effort has been made to determine the timing of garnet growth. The first dating was that of Basu et al. (1989), who used plagioclase-hornblende-garnet to produce a Sm/Nd isochron that yielded an age of 1059 ± 19 Ma. Mezger et al. (1992) conducted their own Sm/Nd investigation using hornblende and the drilled core of a 50 cm garnet to produce an isochron age of 1051 ± 4 Ma. Connelly (2006) utilized seven different fractions of a Gore Mountain garnet to obtain a Lu-Hf isochron age of 1046.6 ± 6 Ma. It is therefore concluded with confidence that the garnets formed at 1049 ± 5 Ma, the average of the three determinations. This is also the local age of peak metamorphism in the 1090–1040 Ma Ottawan phase of the Grenvillian orogeny and serves as a critical data point in ascertaining the evolution of the megacrystic garnet deposits.[50]
Uses
[edit]

Gemstones
[edit]Red garnets were the most commonly used gemstones in the Late Antique Roman world, and the Migration Period art of the "barbarian" peoples who took over the territory of the Western Roman Empire. They were especially used inlaid in gold cells in the cloisonné technique, a style often just called garnet cloisonné, found from Anglo-Saxon England, as at Sutton Hoo, to the Black Sea. Thousands of Tamraparniyan gold, silver and red garnet shipments were made in the old world, including to Rome, Greece, the Middle East, Serica and Anglo Saxons; recent findings such as the Staffordshire Hoard and the pendant of the Winfarthing Woman skeleton of Norfolk confirm an established gem trade route with South India and Tamraparni (ancient Sri Lanka), known from antiquity for its production of gemstones.[51][52][53]
Pure crystals of garnet are still used as gemstones. The gemstone varieties occur in shades of green, red, yellow, and orange.[54] In the United States it is known as the birthstone for January.[2][55][56] It is also the birthstone of Aquarius and Capricorn in tropical astrology.[57][58] The garnet family is one of the most complex in the gem world. It is not a single species, but is composed of multiple species and varieties.[59]
Almandine garnet is the state mineral of Connecticut,[60][61] star garnet is the state gemstone of Idaho,[62] garnet is the state gemstone of New York,[63][64] and grossular garnet is the state gemstone of Vermont.[65]
Industrial uses
[edit]Garnet sand is a good abrasive, and a common replacement for silica sand in sand blasting. Alluvial garnet grains which are rounder are more suitable for such blasting treatments. Mixed with very high pressure water, garnet is used to cut steel and other materials in water jets. For water jet cutting, garnet extracted from hard rock is suitable since it is more angular in form, therefore more efficient in cutting.[66]
Garnet paper is favored by cabinetmakers for finishing bare wood.[67]
Garnet sand is also used for water filtration media.
As an abrasive, garnet can be broadly divided into two categories; blasting grade and water jet grade. The garnet, as it is mined and collected, is crushed to finer grains; all pieces which are larger than 60 mesh (250 micrometers) are normally used for sand blasting. The pieces between 60 mesh (250 micrometers) and 200 mesh (74 micrometers) are normally used for water jet cutting. The remaining garnet pieces that are finer than 200 mesh (74 micrometers) are used for glass polishing and lapping. Regardless of the application, the larger grain sizes are used for faster work and the smaller ones are used for finer finishes.[68]
There are different kinds of abrasive garnets which can be divided based on their origin. The largest source of abrasive garnet today is garnet-rich beach sand which is quite abundant on Indian and Australian coasts and the main producers today are Australia and India.[69]
This material is particularly popular due to its consistent supplies, huge quantities and clean material. The common problems with this material are the presence of ilmenite and chloride compounds. Since the material has been naturally crushed and ground on the beaches for past centuries, the material is normally available in fine sizes only. Most of the garnet at the Tuticorin beach in south India is 80 mesh, and ranges from 56 mesh to 100 mesh size.[citation needed]
River garnet is particularly abundant in Australia. The river sand garnet occurs as a placer deposit.[70]
Rock garnet is perhaps the garnet type used for the longest period of time. This type of garnet is produced in America, China and western India. These crystals are crushed in mills and then purified by wind blowing, magnetic separation, sieving and, if required, washing. Being freshly crushed, this garnet has the sharpest edges and therefore performs far better than other kinds of garnet. Both the river and the beach garnet suffer from the tumbling effect of hundreds of thousands of years which rounds off the edges. Gore Mountain Garnet from Warren County, New York, USA, is a significant source of rock garnet for use as an industrial abrasive.[3]
See also
[edit]References
[edit]- ^ Warr, L. N. (2021). "IMA–CNMNC approved mineral symbols". Mineralogical Magazine. 85 (3): 291–320. Bibcode:2021MinM...85..291W. doi:10.1180/mgm.2021.43. S2CID 235729616.
- ^ a b c Gemological Institute of America, GIA Gem Reference Guide 1995, ISBN 0-87311-019-6
- ^ a b c d e f g h i j k l m n Klein, Cornelis; Hurlbut, Cornelius S. Jr. (1993). Manual of mineralogy : (after James D. Dana) (21st ed.). New York: Wiley. pp. 451–454. ISBN 047157452X.
- ^ pomegranate. Online Etymology Dictionary. Retrieved on 2011-12-25.
- ^ garnet. Online Etymology Dictionary. Retrieved on 2011-12-25.
- ^ Brown, Richard (1995). Ancient astrological gemstones & talismans : the complete science of planetary gemology. Bangkok: A.G.T. Co. p. 47. ISBN 974-89022-4-2. OCLC 33190408.
- ^ Klein & Hurlbut 1993, p. 600.
- ^ Galoisy, L. (1 December 2013). "Garnet: From Stone to Star". Elements. 9 (6): 453–456. Bibcode:2013Eleme...9..453G. doi:10.2113/gselements.9.6.453.
- ^ a b Schmetzer, Karl; Bernhardt, Heinz-Jürgen (Winter 1999). "Garnets from Madagascar with a color change from blue-green to purple" (PDF). Gems & Gemology. 35 (4): 196–201. doi:10.5741/GEMS.35.4.196. Archived (PDF) from the original on 2022-10-09. Retrieved 7 December 2020.
- ^ Baxter, Ethan F.; Caddick, Mark J.; Ague, Jay J. (1 December 2013). "Garnet: Common Mineral, Uncommonly Useful". Elements. 9 (6): 415–419. Bibcode:2013Eleme...9..415B. doi:10.2113/gselements.9.6.415.
- ^ Smyth, Joe. "Mineral Structure Data". Garnet. University of Colorado. Archived from the original on 2007-01-16. Retrieved 2007-01-12.
- ^ Nesse, William D. (2000). Introduction to mineralogy. New York: Oxford University Press. p. 311. ISBN 9780195106916.
- ^ Deer, W. A.; Howie, R. A.; Zussman, J. (2013). "Garnet Group". An Introduction to the Rock-Forming Minerals. Mineralogical Society of Great Britain and Ireland. ISBN 9780903056434.
- ^ Perec, Andrzej (1 October 2017). "Disintegration and recycling possibility of selected abrasives for water jet cutting". DYNA. 84 (203): 249–256. doi:10.15446/dyna.v84n203.62592.
- ^ D. B. Hoover, B. Williams, C. Williams and C. Mitchell, Magnetic susceptibility, a better approach to defining garnets Archived 2011-10-05 at the Wayback Machine, The Journal of Gemmology, 2008, Volume 31, No. 3/4 pp. 91–103
- ^ a b c Lytvynov, L. A. (2011). "On the words used as names for ruby and sapphire" (PDF). Functional Materials. 18 (2): 274–277. Retrieved 7 December 2020.
- ^ Jensen, David E. (November 1975). "The Garnet Group". Rocks & Minerals. 50 (10): 584–587. Bibcode:1975RoMin..50..584J. doi:10.1080/00357529.1975.11767172.
- ^ Nesse 2000, pp. 312, 320.
- ^ "Almandine". Dictionary of Gems and Gemology. 2009. pp. 19–20. doi:10.1007/978-3-540-72816-0_532. ISBN 978-3-540-72795-8.
- ^ Hausel, W. Dan (2000). Gemstones and Other Unique Rocks and Minerals of Wyoming – Field Guide for Collectors. Laramie, Wyoming: Wyoming Geological Survey. pp. 268 p.
- ^ a b Schlegel, Dorothy M. (1957). "Gem stones of the United States". U.S. Geological Survey Bulletin. 1042-G: 203. Bibcode:1957usgs.rept....3S. doi:10.3133/b1042G.
- ^ Klein & Hurlbut 1993, pp. 453, 587–588.
- ^ Nesse 2000, p. 312.
- ^ Schmetzer, Karl; Bernhardt, Heinz-Jürgen (2002). "Gem-quality spessartine-grossular garnet of intermediate composition from Madagascar". Journal of Gemmology. 28 (4): 235–239. doi:10.15506/JoG.2002.28.4.235.
- ^ "Color Change Garnet Value, Price, and Jewelry Information - Gem Society". International Gem Society. Retrieved 2022-10-13.
- ^ Krambrock, K.; Guimarães, F. S.; Pinheiro, M. V. B.; Paniago, R.; Righi, A.; Persiano, A. I. C.; Karfunkel, J.; Hoover, D. B. (July 2013). "Purplish-red almandine garnets with alexandrite-like effect: causes of colors and color-enhancing treatments". Physics and Chemistry of Minerals. 40 (7): 555–562. Bibcode:2013PCM....40..555K. doi:10.1007/s00269-013-0592-6. S2CID 95448333.
- ^ Colophonite (a variety of Andradite): information about the mineral colophonite in the Mindat database.
- ^ Saha, Abhishek; Ray, Jyotisankar; Ganguly, Sohini; Chatterjee, Nilanjan (10 July 2011). "Occurrence of melanite garnet in syenite and ijolite–melteigite rocks of Samchampi–Samteran alkaline complex, Mikir Hills, Northeastern India". Current Science. 101 (1): 95–100. JSTOR 24077869.
- ^ Plümper, Oliver; Beinlich, Andreas; Bach, Wolfgang; Janots, Emilie; Austrheim, Håkon (September 2014). "Garnets within geode-like serpentinite veins: Implications for element transport, hydrogen production and life-supporting environment formation". Geochimica et Cosmochimica Acta. 141: 454–471. Bibcode:2014GeCoA.141..454P. doi:10.1016/j.gca.2014.07.002.
- ^ Coombs, D. S.; Kawachi, Y.; Houghton, B. F.; Hyden, G.; Pringle, I. J.; Williams, J. G. (August 1977). "Andradite and andradite-grossular solid solutions in very low-grade regionally metamorphosed rocks in Southern New Zealand". Contributions to Mineralogy and Petrology. 63 (3): 229–246. Bibcode:1977CoMP...63..229C. doi:10.1007/BF00375574. S2CID 129908263.
- ^ Phillips, Wm. Revell; Talantsev, Anatoly S. (Summer 1996). "Russian demantoid, czar of the garnet family" (PDF). Gems & Gemology. 32 (2): 100–111. doi:10.5741/GEMS.32.2.100. Archived (PDF) from the original on 2022-10-09. Retrieved 7 December 2020.
- ^ Modreski, Peter J. (1 February 1993). "Featured Mineral Group at the 1993 Tucson Show: Garnet". Rocks & Minerals. 68 (1): 20–33. Bibcode:1993RoMin..68...20M. doi:10.1080/00357529.1993.9926521.
- ^ Mindat.org - Tsavorite
- ^ Feneyrol, J.; Giuliani, G.; Ohnenstetter, D.; Fallick, A.E.; Martelat, J.E.; Monié, P.; Dubessy, J.; Rollion-Bard, C.; Le Goff, E.; Malisa, E.; Rakotondrazafy, A.F.M.; Pardieu, V.; Kahn, T.; Ichang'i, D.; Venance, E.; Voarintsoa, N.R.; Ranatsenho, M.M.; Simonet, C.; Omito, E.; Nyamai, C.; Saul, M. (September 2013). "New aspects and perspectives on tsavorite deposits". Ore Geology Reviews. 53: 1–25. Bibcode:2013OGRv...53....1F. doi:10.1016/j.oregeorev.2013.01.016.
- ^ Nixon, Peter H.; Hornung, George (1968). "A new chromium garnet end member, knorringite, from Kimberlite". American Mineralogist. 53 (11–12): 1833–1840. Retrieved 7 December 2020.
- ^ S. Geller Crystal chemistry of the garnets Zeitschrift für Kristallographie, 125(125), pp. 1–47 (1967) doi:10.1524/zkri.1967.125.125.1
- ^ Yariv, Amnon (1989). Quantum Electronics (3rd ed.). Wiley. pp. 208–211. ISBN 978-0-471-60997-1.
- ^ Teikemeier, G; Goldberg, DJ (1997). "Skin resurfacing with the erbium:YAG laser". Dermatologic Surgery. 23 (8). Philadelphia: Lippincott Williams & Wilkins: 685–687. doi:10.1111/j.1524-4725.1997.tb00389.x. PMID 9256915. S2CID 31557815.
- ^ Bornstein, E (2004). "Proper use of Er:YAG lasers and contact sapphire tips when cutting teeth and bone: scientific principles and clinical application". Dentistry Today. 23 (83): 86–89. PMID 15354712.
- ^ Kokavec, Jan; Wu, Zhichao; Sherwin, Justin C; Ang, Alan JS; Ang, Ghee Soon (2017-06-01). "Nd:YAG laser vitreolysis versus pars plana vitrectomy for vitreous floaters". The Cochrane Database of Systematic Reviews. 2017 (6): CD011676. doi:10.1002/14651858.CD011676.pub2. ISSN 1469-493X. PMC 6481890. PMID 28570745.
- ^ "What is YIG and How Does It Work So Well?". www.microlambdawireless.com. Retrieved 2023-07-17.
- ^ Moore, Cheryl (2015). "Towards a Greater Understanding of Hydrothermally Grown Garnets and Sesquioxide Crystals for Laser Applications". Clemson University Tiger Prints. Bibcode:2015PhDT.......308M.
- ^ "Lutetium Aluminum Garnet - LuAG - Lu3Al5O12". scientificmaterials.com. Retrieved 2016-04-29.
- ^ Majeed, Hassaan; Shaheen, Amrozia; Anwar, Muhammad Sabieh (2013). "Complete Stokes polarimetry of magneto-optical Faraday effect in a terbium gallium garnet crystal at cryogenic temperatures". Optics Express. 21 (21). Washington, D.C.: The Optical Society: 25148–25158. Bibcode:2013OExpr..2125148M. doi:10.1364/OE.21.025148. PMID 24150356.[permanent dead link]
- ^ Nesse, William D. (2013). Introduction to Optical Mineralogy (International Fourth ed.). New York: Oxford University Press. pp. 252–255. ISBN 978-0-19-984628-3.
- ^ a b c d Klein, C; Hurlbut, C. D. (1985). Manual of Mineralogy. New York: John Wiley and Sons. pp. 375–378. ISBN 0-471-80580-7.
- ^ a b c "P-T-t Paths". Teaching Phase Equilibria. Retrieved 2020-03-19.
- ^ Seman, S.; Stockli, D. F.; McLean, N. M. (2017-06-05). "U-Pb geochronology of grossular-andradite garnet". Chemical Geology. 460: 106–116. Bibcode:2017ChGeo.460..106S. doi:10.1016/j.chemgeo.2017.04.020. ISSN 0009-2541.
- ^ Blackburn, Terrence J.; Stockli, Daniel F.; Carlson, Richard W.; Berendsen, Pieter (2008-10-30). "(U–Th)/He dating of kimberlites—A case study from north-eastern Kansas". Earth and Planetary Science Letters. 275 (1): 111–120. Bibcode:2008E&PSL.275..111B. doi:10.1016/j.epsl.2008.08.006. ISSN 0012-821X.
- ^ a b McLelland, James M.; Selleck, Bruce W. (2011). "Megacrystic Gore Mountain–type garnets in the Adirondack Highlands: Age, origin, and tectonic implications". Geosphere. 7 (5). Geological Society of America: 1194–1208. Bibcode:2011Geosp...7.1194M. doi:10.1130/GES00683.1.
- ^ "Staffordshire Hoard Festival 2019". The Potteries Museum & Art Gallery. Archived from the original on 18 June 2019. Retrieved 18 June 2019.
- ^ "A trail of garnet and gold: Sri Lanka to Anglo-Saxon England". The Historical Association. 22 June 2017. Retrieved 18 June 2019.
- ^ "Acquisitions of the month: June 2018". Apollo Magazine. 5 July 2018. Retrieved 18 June 2019.
- ^ Geological Sciences at University of Texas, Austin. Geo.utexas.edu. Retrieved on 2011-12-25.
- ^ "Tips & Tools: Birthstones". The National Association of Goldsmiths. Archived from the original on 2007-05-28. Retrieved 2014-06-16.
- ^ Kunz, George F. (1913). The curious lore of precious stones. Lippincott. pp. 275–306, pp. 319-320
- ^ Knuth, Bruce G. (2007). Gems in Myth, Legend and Lore (Revised edition). Parachute: Jewelers Press. p. 294.
- ^ Kunz (1913), pp. 345–347
- ^ "Garnet Value, Price, and Jewelry Information". International Gem Society. Retrieved 2021-11-16.
- ^ "State of Connecticut – Sites, Seals and Symbols". State of Connecticut. Retrieved 2009-11-12.
- ^ State of Connecticut, Sites º Seals º Symbols Archived 2008-07-31 at the Wayback Machine; Connecticut State Register & Manual; retrieved on December 20, 2008
- ^ "Idaho Symbols". State of Idaho. Archived from the original on 2010-06-30. Retrieved 2009-11-12.
- ^ "Minerals of New York State". State of New York. Retrieved 2022-02-25.
- ^ New York State Gem Archived 2007-12-08 at the Wayback Machine; State Symbols USA; retrieved on October 12, 2007
- ^ "Vermont Emblems". State of Vermont. Archived from the original on 2009-10-29. Retrieved 2009-11-12.
- ^ Rapple, R. Randolph. "Selecting the right waterjet abrasive". The Fabricator. Retrieved 17 July 2023.
- ^ Joyce, Ernest (1987) [1970]. Peters, Alan (ed.). The Technique of Furniture Making (4th ed.). London: Batsford. ISBN 071344407X.
- ^ AlphaVariable. "Garnet". AlphaVariable. Retrieved 2024-08-08.
- ^ Briggs, J. (2007). The Abrasives Industry in Europe and North America. Materials Technology Publications. ISBN 978-1-871677-52-2.
- ^ "Industrial Mineral Opportunities in New South Wales" (PDF). Archived from the original (PDF) on 2014-06-22. Retrieved 2014-11-06.
Further reading
[edit]- Hurlbut, Cornelius S.; Klein, Cornelis, 1985, Manual of Mineralogy, 20th ed., Wiley, ISBN 0-471-80580-7
- Color Encyclopedia of Gemstones, ISBN 0-442-20333-0
External links
[edit]- http://www.gemstonemagnetism.com contains a comprehensive section about garnets and garnet magnetism.
- USGS Garnet locations – USA
- http://gemstone.org/education/gem-by-gem/154-garnet
- http://www.mindat.org/min-10272.html
- Blog post on garnets on the Law Library of Congress's blog
- https://www.birthstone.guide/garnet-birthstone-meaning Garnet birthstone stories