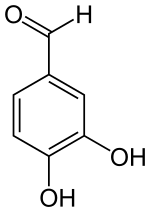
3,4-Dihydroxybenzaldehyde
Appearance
(Redirected from Protocatechuic aldehyde)
| |
| Names | |
|---|---|
| Preferred IUPAC name
3,4-Dihydroxybenzaldehyde | |
| Other names
Protocatechuic aldehyde
Protocatechualdehyde Rancinamycin IV | |
| Identifiers | |
3D model (JSmol)
|
|
| 774381 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.004.889 |
| EC Number |
|
| 123001 | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H6O3 | |
| Molar mass | 138.12 g/mol |
| Related compounds | |
Related compounds
|
2,4-Dihydroxybenzaldehyde |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
3,4-Dihydroxybenzaldehyde, also known as protocatechuic aldehyde, is a phenolic aldehyde, a compound released from cork stoppers into wine.[1] It is an isomer of 2,4-dihydroxybenzaldehyde.
This molecule can be used as a precursor in the vanillin synthesis by biotransformation by cell cultures of Capsicum frutescens, a type of Chili pepper.[2] It is also found in the mushroom Phellinus linteus.[3]
Pharmacological effects
[edit]Protocatechuic aldehyde regulates G protein-coupled estrogen receptor-1 (GPER-1) and exhibits protective effects in endothelial dysfunction and atherosclerosis.[4]
References
[edit]- ^ Conde E, Cadahía E, García-Vallejo MC, Fernández de Simón B (August 1998). "Polyphenolic Composition of Quercus suber Cork from Different Spanish Provenances". Journal of Agricultural and Food Chemistry. 46 (8): 3166–71. doi:10.1021/jf970863k.
- ^ Rao SR, Ravishankar GA (January 2000). "Biotransformation of protocatechuic aldehyde and caffeic acid to vanillin and capsaicin in freely suspended and immobilized cell cultures of Capsicum frutescens". Journal of Biotechnology. 76 (2–3): 137–46. doi:10.1016/s0168-1656(99)00177-7. PMID 10656328. S2CID 28017446.
- ^ Lee YS, Kang YH, Jung JY, Lee S, Ohuchi K, Shin KH, et al. (October 2008). "Protein glycation inhibitors from the fruiting body of Phellinus linteus". Biological & Pharmaceutical Bulletin. 31 (10): 1968–72. doi:10.1248/bpb.31.1968. PMID 18827365.
- ^ Kong BS, Cho YH, Lee EJ (2014). "G protein-coupled estrogen receptor-1 is involved in the protective effect of protocatechuic aldehyde against endothelial dysfunction". PLOS ONE. 9 (11): e113242. Bibcode:2014PLoSO...9k3242K. doi:10.1371/journal.pone.0113242. PMC 4239058. PMID 25411835.
