Plagiolophus (mammal)
| Plagiolophus | |
|---|---|

| |
| Plagiolophus minor skeleton from Baden-Württemberg, State Museum of Natural History Stuttgart | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Perissodactyla |
| Family: | †Palaeotheriidae |
| Subfamily: | †Palaeotheriinae |
| Genus: | †Plagiolophus Pomel, 1847 |
| Type species | |
| †Plagiolophus minor Cuvier, 1804
| |
| Other species | |
For subspecies suggested, see below. | |
| Synonyms | |
|
Genus synonymy
Synonyms of P. minor
Synonyms of P. ovinus
Synonyms of P. annectens
Synonyms of P. javali
Synonyms of P. lugdunensis
Synonyms of P. major
Dubious species
| |
Plagiolophus (Ancient Greek: πλαγιοϛ (oblique) + λοφος (crest) meaning "oblique crest") is an extinct genus of equoids belonging to the family Palaeotheriidae. It lived in Europe from the middle Oligocene to the early Oligocene. The type species P. minor was initially described by the French naturalist Georges Cuvier in 1804 based on postcranial material including a now-lost skeleton originally from the Paris Basin. It was classified to Palaeotherium the same year but was reclassified to the subgenus Plagiolophus, named by Auguste Pomel in 1847. Plagiolophus was promoted to genus rank by subsequent palaeontologists and today includes as many as seventeen species. As proposed by the French palaeontologist Jean A. Remy in 2004, it is defined by three subgenera: Plagiolophus, Paloplotherium, and Fraasiolophus.
Plagiolophus is an evolutionarily derived member of its family with tridactyl (or three-toed) forelimbs and hindlimbs. It has longer postcanine diastemata (gaps between teeth) compared to Palaeotherium and brachyodont (low-crowned) dentition that evolutionarily progressed towards hypsodonty (high-crowned) in response to climatic trends. It is also defined in part by an elongated facial region, deep nasal notch, and orbits for the eyes that are more positioned backwards compared to those of Palaeotherium. Plagiolophus, as a species-rich genus, has a wide body mass range extending from less than 10 kg (22 lb) in the smallest species P. minor to over 150 kg (330 lb) in the largest species P. javali. The postcranial builds of several species suggest that some had stockier body builds (P. annectens, P. fraasi, P. javali) while some others were lightly built for cursorial (running) adaptations (P. minor, P. ministri, P. huerzeleri).
Plagiolophus and other members of the Palaeotheriinae likely descended from the earlier subfamily Pachynolophinae in the middle Eocene. Western Europe, where Plagiolophus was largely present, was an archipelago that was isolated from the rest of Eurasia, meaning that it lived in an environment with various other faunas that also evolved with strong levels of endemism. While many species had short temporal ranges, P. minor was long-lasting to the extent that researchers observed trends in changes in its dietary habits. More specifically, P. minor over time was observed to have consumed less hard foods (fruits, seeds) and became more specialized but less selective towards tough, abrasive, and older leaves in response to environmental trends in the late Eocene to early Oligocene. Its dietary habits would have allow it to niche partition with other palaeotheres like Palaeotherium and Leptolophus. Plagiolophus was consistently diverse for much of its evolutionary history and survived far past the Grande Coupure extinction event, likely because some of its species were well-adapted towards major environmental trends as a result of their dietary changes and cursorial nature. It was able to adapt to more seasonal climates after the Grande Coupure and coexisted with immigrant faunas from the faunal turnover event. Its eventual extinction by the later early Oligocene marked the complete extinction of the Palaeotheriidae.
Taxonomy
[edit]Research history
[edit]Early history
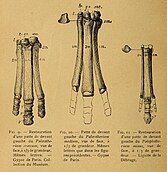
[edit]In 1804, the French naturalist Georges Cuvier, having established the genus Palaeotherium and some of its species (P. medium and P. magnum), recognized a third species Palaeotherium minus based on some postcranial fossils from the gypsum quarries of the outskirts of Paris (known as the Paris Basin), although he did not elaborate further on them.[1] In a later journal of the same year, he described a nearly completely skeleton from the quarries of the French commune of Pantin, originally found by the French naturalist Auguste Nicholas de Saint-Genis. According to Cuvier, the quarry workers previously thought the skeleton to be of a ram, and it was presented as such in public newspapers. The French prefect Nicolas Frochot later acquired it and brought it to the National Museum of Natural History, France, where Cuvier was then able to observe that it must have been a skeleton of a Palaeotherium species. He noted that the majority of the fossil bones were detached from others and/or damaged but that postcranial fossils such as scapulae, humeri, femora, vertebrae, and ribs were found. The naturalist also provided a figure of the skeleton within the journal.[2]
In 1812, Cuvier published published his drawing of a skeletal reconstruction of P. minus based on known fossil remains of the species including the mostly complete skeleton. He also suggested theoretical lifestyles of several Palaeotherium species. In particular, he suggested that P. minus resembled a tapir, was smaller than a sheep, and was cursorial based on the slender morphologies of its leg bones. Such a behaviour and small size would have differed from other species of Palaeotherium, several of which according to Cuvier had stockier limb bone builds. He also identified that P. medium, P. magnum, P. minus, P. crassum, and P. curtum were all tridactyl, or three-toed.[3][4]

"P. minus" (= Plagiolophus minor) was amongst the fossil mammal species represented as sculptures in the Crystal Palace Dinosaurs attraction in the Crystal Palace Park in the United Kingdom, open to the public since 1854 and constructed by English sculptor Benjamin Waterhouse Hawkins. The Plagiolophus sculpture is smaller than the P. magnum and P. medium sculptures and is in a sitting position unlike the other two. The models' resemblances to tapirs reflected early perceptions that the palaeothere species resembled them in body plan appearances. Despite this, the sculptures differ from living tapirs in several ways, such as shorter plus taller faces, higher eye positions, slender legs, longer tails, and the presence of three toes on the forelimbs unlike the four toes of the forelimbs of tapirs.[5]
Hawkins and other workers seemingly used Cuvier's research for reference to the anatomy of P. minor and reproduced its size and proportions accordingly. The P. minor sculpture, sheep-sized, originally had a short head that probably measured about 1.3 m (4 ft 3 in) in length and had pointed ears, large eyes, long lips, a stocky proboscis, a muscular neck, and a short plus slender tail. It looks similar to the P. medium sculpture overall but lacks skin details. Although the original head's form is poorly known, it appeared to have been longer and more robust than that of P. medium. Within the later half of the 20th century, the original head was lost and replaced with a head cast of P. medium. Because the size and form of the head made it difficult to attach to the P. minor body normally, the back portion of the cranium was removed and the neck lengthened. This resulted in the sculpture appearing to look forward instead of upwards like before. The P. minor sculpture lost its head twice more, once recently in 2014 when its head was tossed into a lake of the Crystal Palace by an unknown criminal and had to be recovered.[5]
Later 19th century research history
[edit]
In 1846, French palaeontologist Auguste Aymard recorded a mandible with teeth from the French department of Haute-Loire, noting that it was the approximate size of that of Palaeotherium curtum but had different molar morphologies from it and the small-sized "P. minus", establishing the name P. ovinum.[6] The year after in 1847, the French palaeontologist Auguste Pomel erected the Palaeotherium subgenus Plagiolophus, which he reclassified P. minus to.[7] The genus name derives in Ancient Greek from πλαγιοϛ ("oblique") and λοφος ("crest") meaning "oblique crest".[8]
British palaeontologist Richard Owen in 1848 wrote about a nearly complete lower jaw with both deciduous and permanent dental sets that was uncovered from the Eocene beds of Hordle, England by Alexander Pytts Falconer, observing that it had one less premolar for a total of 3 of them than in other species of Palaeotherium and erecting the genus Paloplotherium based on the mandible. He then described a cranium belonging to Paloplotherium that similarly had nearly complete dentition but evolutionarily lost a premolar. After comparing the dentition to those of both Palaeotherium and Anoplotherium, he determined that the dentition of Paloplotherium was similar to that of the former but differed mainly by the absence of the first premolar. He wrote that the permanent dental formula of Paloplotherium is 3.1.3.33.1.3.3 for a total of 40 teeth and erected the species Paloplotherium annectens.[9] Paloplotherium derives from the Ancient Greek words παλαιός ("ancient"), ὅπλον ("arms"), and θήρ ("wild beast") meaning "ancient armed beast".[8]

In 1852, German palaeontologist Christian Erich Hermann von Meyer, recognizing Plagiolophus as a distinct genus and emending Plagiolophus minus to Plagiolophus minor, erected the species P. fraasi based on fossils from the German locality of Frohnstetten originally found by Oscar Fraas.[10] Fraas had studied fossils of palaeotheres from Frohnstetten since 1851, assembling a complete skeleton of P. minor using fossils from there in 1853.[11][12] In 1853, Pomel listed in the genus Plagiolophus multiple previously recognized species, namely P. ovinus (reclassified from Palaeotherium and emended from P. ovinum), P. minor, and P. annectens (by extent synonymizing Paloplotherium with Plagiolophus). He also erected another species P. tenuirostris.[13] In 1862, Swiss palaeontologist Ludwig Ruetimeyer established P. minutus based on dental remains from the Swiss locality of Egerkingen.[14]
Not all taxonomists agreed on Paloplotherium as a synonymous genus. In 1865 for example, French palaeontologist Jean Albert Gaudry recognized Paloplotherium as valid genus instead of Plagiolophus, grouping P. minor, P. ovinus, and P. annectens into it and erecting another species P. codiciense.[15] In 1869, Swiss palaeontologists François Jules Pictet de la Rive and Aloïs Humbert wrote that Palaeotherium, Paloplotherium, and Plagiolophus were all valid genera and erected two species for the latter: P. siderolithicus using fossil molars from a museum collection and P. valdensis based on a mandible that was smaller in proportion than that of P. minor.[16] In 1877, French naturalist Henri Filhol erected Paloplotherium Javalii based on fossil jaws including that from the fossil collection of the French official Ernest Javal, who he named the species after.[a][17] Ruetimeyer in 1891 erected another species Paloplotherium magnum, stating that its size based on fossil material would have been that of Palaeotherium magnum.[18]
20th-21st century taxonomy
[edit]
In 1901, researchers Charles Depéret and G. Carrière designated the species name Paloplotherium lugdunense to fossil material originally from the fossil deposits from the French commune of Lissieu. They said that the species was barely larger than P. codiciense and that it was also known from the locality of Robiac.[19] The year after in 1902, Swiss palaeontologist Hans Georg Stehlin erected Paloplotherium Rütimeyeri, but he only wrote that it was known from Egerkingen and did not elaborate further on it.[20] In 1904, Swiss palaeontologist Hans Georg Stehlin synonymized Paloplotherium magnum with Palaeotherium castrense and erected two species of Plagiolophus: P. Nouleti from a fossil mandible from the French commune of Viviers-lès-Montagnes and P. Cartailhaci using fossils from the commune of Peyregoux.[21] In one of his monographies, written the same year, Stehlin erected Palaeotherium Rütimeyeri with official fossil descriptions to replace the previous species name and synonymized Paloplotherium javali with Plagiolophus fraasi. He also erected the species P. cartieri based on Egerkingen fossils, arguing that its size was between P. annectens and P. minor plus that its fossils resembled those of P. codidiciensis.[22] In 1917, French palaeontologist Charles Depéret erected the species P. Oweni (also recognizing it by the name P. annectens mut. Oweni from fossils in the commune of Gargas, arguing that it was a more advanced species of Plagiolophus based on the size and morphology of its premolars. He also reclassified "Paloplotherium" codiciense into its own genus Paraplagiolophus.[23] In 1965, French palaeontologist Jean Albert Remy erected the genus Leptolophus, reclassifying P. nouleti into the taxon.[24]
In 1986, British palaeontologist Jerry J. Hooker listed Paloplotherium as a synonym of Palaeotherium and listed P. minor, P. cartieri, P. lugdunensis (emended name), P. cartailhaci, P. annectens, P. fraasi, and P. javalii as valid species, although he doubted that P. javalii was distinct from P. fraasi. He also erected P. curtisi using fossils from fragmentary cranial remains from the Barton Beds of the United Kingdom and recognized two subspecies: P. curtisi curtisi and P. curtisi creechensis. The species was named after an individual named R.J. Curtis, who found the specimens for the former subspecies.[25] In 1989, palaeontologists Michel Brunet and Yves Jehenne considered Paloplotherium to be distinct from Palaeotherium and erected for the former genus two additional species: P. majus from the fossil collections of the Quercy Phosphorites Formation and P. ministri from the French commune of Villebramar.[26] Remy in 1994, however, rejected the claim by Brunet and Jehenne that Paloplotherium was a distinct genus from Plagiolophus, instead suggesting to convert the former into a Plagiolophus subgenus.[27]
In 1994, Spanish palaeontologist Miguel Ángel Cuesta Ruiz-Colmenares erected two Plagiolophus species, the first being P. casasecaensis, named after the Spanish municipality of Casaseca de Campeán within the Duero Basin. The second species he recognized was P. mazateronensis, also from the Duero Basin; it was named after the Mazaterón province in the municipality of Soria.[28] In 1997, another Spanish palaeontologist Lluís Checa Soler analyzed a dental specimen, stating his belief that it belonged to Plagiolophus and that the species would be defined by its smaller size and primitive characteristics compared to other species. He proposed the name P. plesiomorphicus but sought to not formally define it until more complete material assigned to the species was found.[29]
In 2000, Remy described a skull of a male Plagiolophus individual that was within a sandstone block originally from the French department of Vaucluse, assigning it the new species name P. huerzeleri. The species was named after Johannes Hürzeler, Swiss palaeontologist and former director of the oteology department of the Natural History Museum of Basel. Remy had also emended P. majus to P. major and suggested both Plagiolophus and Paloplotherium as valid subgenera for Plagiolophus.[30] Remy, in 2004, followed up by erecting P. ringeadei, named after Ruch fossil deposit discoverer Michel Ringead and known by a skull of an adult female with cheek teeth, and P. mamertensis, which was assigned a left maxilla with partial dentition from Robiac for a holotype specimen. He also listed P. minutus and P. plesiomorphicus both as nomen dubia (doubtful taxon names). Remy reiterated both Plagiolophus and Paloplotherium as defined subgenera for Plagiolophus and created a third subgenus Fraasiolophus.[31]
Classification
[edit]
Plagiolophus belongs to the Palaeotheriidae, largely considered to be one of two major hippomorph families in the superfamily Equoidea, the other being the Equidae. Alternatively, some authors have proposed that equids are more closely related to the Tapiromorpha than to the Palaeotheriidae. It is also usually thought to consist of two families, the Palaeotheriinae and Pachynolophinae; a few authors alternatively have argued that pachynolophines are more closely related to other perissodactyl groups than to palaeotheriines.[32] Some authors have also considered the Plagiolophinae to be a separate subfamily, while others group its genera into the Palaeotheriinae.[33] Plagiolophus has also been suggested to belong to the tribe Plagiolophini, one of three proposed tribes within the Palaeotheriinae along with the Leptolophini and Palaeotheriini.[31] The Eurasian distribution of the palaeotheriids (or palaeotheres) were in contrast to equids, which are generally thought to have been an endemic radiation in North America. Some of the most basal equoids of the European landmass are of uncertain affinities, with some genera being thought to potentially belong to the Equidae.[34] Palaeotheriids are well-known for having lived in western Europe during much of the Palaeogene but were also present in eastern Europe, possibly the Middle East, and, in the case of pachynolophines (or pachynolophs), Asia.[32][33]
The Perissodactyla makes its earliest known appearance in the European landmass in the MP7 faunal unit of the Mammal Palaeogene zones. During the temporal unit, many genera of basal equoids such as Hyracotherium, Pliolophus, Cymbalophus, and Hallensia made their first appearances there. A majority of the genera persisted to the MP8-MP10 units, and pachynolophines such as Propalaeotherium and Orolophus arose by MP10.[34][35] The MP13 unit saw the appearances of later pachynolophines such as Pachynolophus and Anchilophus along with definite records of the first palaeotheriines such as Palaeotherium and Paraplagiolophus.[36] The palaeotheriine Plagiolophus has been suggested to have potentially made an appearance by MP12. It was by MP14 that the subfamily proceeded to diversify,[37] and the pachynolophines were generally replaced but still reached the late Eocene. In addition to more widespread palaeothere genera such as Plagiolophus, Palaeotherium, and Leptolophus, some of their species reaching medium to large sizes, various other palaeothere genera that were endemic to the Iberian Peninsula, such as Cantabrotherium, Franzenium, and Iberolophus, appeared by the middle Eocene.[36]
The phylogenetic tree for several members of the family Palaeotheriidae within the order Perissodactyla (including three outgroups) as created by Remy in 2017 and followed by Remy et al. in 2019 is defined below:[38][37]
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
As shown in the above phylogeny, the Palaeotheriidae is defined as a monophyletic clade, meaning that it did not leave any derived descendant groups in its evolutionary history. Hyracotherium sensu stricto (in a strict sense) is defined as amongst the first offshoots of the family and a member of the Pachynolophinae. "H." remyi, formerly part of the now-invalid genus Propachynolophus, is defined as a sister taxon to more derived palaeotheriids. Both Pachynolophus and Lophiotherium, defined as pachynolophines, are defined as monophyletic genera. The other pachynolophines Eurohippus and Propalaeotherium consistute a paraphyletic clade in relation to members of the derived and monophyletic subfamily Palaeotheriinae (Leptolophus, Plagiolophus, and Palaeotherium), thus making Pachynolophinae a paraphyletic subfamily clade.[38]
List of lineages
[edit]Unlike Palaeotherium where many species have subspecies, Plagiolophus only has one species with defined subspecies, P. curtisi. All species of Plagiolophus are classified in one of three subgenera. The following table defines the species and subspecies of Plagiolophus and additional information about them:
| Lineage | Proposed subgenus | MP unit(s) | Author(s) of taxon | Taxon publication year |
|---|---|---|---|---|
| P. annectens | Paloplotherium | 16, 17 | Owen | 1848 |
| P. cartailhaci | Paloplotherium | 16 | Stehlin | 1904 |
| P. cartieri | Paloplotherium | 12?, 13, 14 | Stehlin | 1904 |
| P. casasecaensis | Paloplotherium | 13 | Cuesta | 1994 |
| P. curtisi curtisi | Paloplotherium | 16 | Hooker | 1986 |
| P. curtisi creechensis | Paloplotherium | 16 | Hooker | 1986 |
| P. fraasi | Fraasiolophus | 20 | von Meyer | 1852 |
| P. huerzeleri | Plagiolophus | 23 | Remy | 2000 |
| P. javali | Plagiolophus | 25 | Filhol | 1877 |
| P. lugdunensis | Paloplotherium | 14 | Depéret & Carrière | 1901 |
| P. major | Paloplotherium | 20 | Brunet & Jehenne | 1989 |
| P. mamertensis | Paloplotherium | 16 | Remy | 2004 |
| P. mazateronensis | Paloplotherium | 16, 17 | Cuesta | 1994 |
| P. ministri | Plagiolophus | 22 | Brunet & Jehenne | 1989 |
| P. minor | Plagiolophus | 18, 19, 20, 21, 22 | Cuvier | 1804 |
| P. ovinus | Plagiolophus | 21 | Aymard | 1846 |
| P. oweni | Paloplotherium | 18 | Depéret | 1917 |
| P. ringeadei | Plagiolophus | 21 | Remy | 2004 |
Description
[edit]Skull
[edit]
The Palaeotheriidae is diagnosed in part as generally having orbits that are wide open in the back area and are located in the middle of the skull or in a slight frontal area of it. The nasal bones are slightly extensive to very extensive in depth.[41] Plagiolophus is diagnosed in part as having skull lengths that vary by species and range from 170 mm (6.7 in) to 400 m (1,300 ft). It is also defined by many other unique cranial traits, among them being the skull's elongated facial region, especially in later species, that is more well-developed compared to that of Palaeotherium. The maxilla, at the area with the canine, is wide; the muzzle in comparison is thin. The nasal notch, found on the front lower edge of the maxilla, is generally deep, ranges from P1 to M1, and has its lower edges formed from those of the premaxilla and maxilla. The zygomatic arch is narrow and elevates up to the back of the orbit. The mandibular symphysis, the middle of the mandible, is elongated and contains projecting incisors. The horizontal ramus (or body) of the mandible is wide from front to back and has a prominent coronoid process.[31][42] The subgenus Plagiolophus is defined by a shallow nasal notch that is always located in front of P2, the lack of any preorbital fossa and a thinner body of the mandible compared to that of Paloplotherium. Paloplotherium contrasts from Plagiolophus in having a deep nasal notch is always behind P2 and a larger skull size, but the former also shares the lack of any preorbital fossae. Plagiolophus also differs from Paloplotherium in having a thinner horizontal ramus of the mandible. Fraasiolophus differs from the other two subgenera solely by the presence of a deep preorbital fossa.[31]
The skull of Plagiolophus appears slightly triangular in shape, has a maximum width either above or in front of where the mandible articulates with other skull bones, and has a wider front area compared to that of Leptolophus. The skull length of Plagiolophus generally increases over time as part of an evolutionary trend of species. For instance, the skull of P. huerzeleri, one of the latest species to have existed, has a more elongated and skull (making it more equinelike) than that of P. annectens, an earlier-appearing species; the former species also has a longer anterior orbital region, a higher orbit position, implying different arrangements of facial muscles compared to the latter. The orbit of Plagiolophus is slightly behind the midlength of the skull, making its position more similar to that of the Palaeogene equid Mesohippus than the more forward orbit of Palaeotherium. The nasal opening in Plagiolophus is positioned higher than that of Propalaeotherium and varies in form by species, generally becoming less hollow in later species contrary to the evolutionary trends observed in Palaeotherium.[31]

The body of the premaxilla is elongated but low height and hosts all the incisors. The palatine bone stretches up to the lacrimal bone. The optic foramen, located in the sphenoid bone, is larger than that of Palaeotherium; it is separated from other foramen like in other palaeotheres and stretches more forwards compared to equines. Those of P. annectens and P. minor pierce through the skull and connect with each other as part of a single optic canal path; those of P. cartieri and species originating from the Oligocene have thick septa, or anatomical walls that separate them and therefore lead to two different optic canals for each foramen. The sagittal crest (midline of skull's top) and nuchal lines are both well-developed, the latter displaying stronger sexual dimorphism in males. The post-orbital constriction occurs behind the postorbital process like in most other palaeotheres but unlike in Palaeotherium.[31]
The postglenoid process, located in the squamous part of the temporal bone, is large in both Plagiolophus and Palaeotherium and has parallel front and back walls. The process is where the hollowing of the ear canal's roof is located, taking different shapes in different species. The underside of the ear canal does not take a canalized form except in P. huerzeleri. The petrous part of the temporal bone largely contacts the basilar part of the occipital bone and is slightly hollowed.[31]
The horizontal ramus of the manible is robust but varies in such based on factors pertaining to species morphology and sexual dimorphism, its underside being mostly convex but also straight at the front area. The vertical ramus is extensive like in Palaeotherium but is wider at the area of articulation. The condyloid process of the mandible, which articulates with the temporal bone, is narrow, elongated, and sloped. The coronoid process of the mandible is wide like in Palaeotherium but may sometimes be wider; it is able to support temporalis muscles well for chewing.[31]
Dentition
[edit]
Derived palaeotheres are generally diagnosed as having selenolophodont upper molars and selenodont lower molars that are mesodont, or medium-crowned, in height. The canines strongly protrude and are separated from the premolars by medium to long diastemata and from the incisors by short ones in both the upper and lower dentition. The other teeth are paired closely with each other in both the upper and lower rows.[41] Plagiolophus is defined by brachyodont dentitions that became progressively hypsodont (high-crowned) to semi-hypsodont evolutionarily, the premolars being semi-molarized and the molars increasing in size from the front end to the back end of the dental row. The dental formula of Plagiolophus is 3.1.3-4.33.1.3-4.3, totaling at 42 to 44 teeth present.[28][31][40] It differs from Leptolophus is appearing less lophodont and lesser degree of heterodonty in its cheek teeth. Plagiolophus also differs from Paraplagiolophus in having cheek teeth that appear narrower and more lophodont. The postcanine diastemata of Plagiolophus are longer than those of Palaeotherium but display varying degrees of such based on sex and species. The subgenus Plagiolophus differs from Paloplotherium by its longer postcanine diastemata and greater degree of hypsodonty, and the former has proportionally narrow and oblique lingual lophs in its upper cheek teeth compared to that of the latter. The latter also has a stronger degree of heterodonty from its premolars and smaller internal cusps compared to the former.[28]
While not all species of Plagiolophus are currently known by fossil incisors, the incisors of known species reveal a common trait of chisel-like shapes typical of the equoids. The outermost edges of the incisors are of identical lengths but take different forms from each other. The edges of the incisors are sharp and thin, giving them flat appearances. The frontmost incisors, the first incisors, have elongated labial (or front in relation to the mouth) faces that are equal in size to that of Palaeotherium but smaller than that of Leptolophus. The lingual (back) face is shorter than the labial face, takes a concave shape, and is surrounded by a cingulum that ascends up to the outermost edge of the incisor. I1 is inclined and appears to project forward. The second and third incisors have less symmetrical crown shapes compared to the first. Both I2 and I2 have somewhat oblique outermost edges. The third incisors appear to be the most differentiated incisor variants and are the smallest ones. The canines have labial surfaces that are convex compared to their lingual counterparts. The widths of the canines vary because of sexual dimorphism. While the upper canines appear to be inclined forward and outwards due to the positions of their roots, the lower canines and their crowns have straighter positions, although the crowns diverge as well.[31]

The oldest species of Plagiolophus had four upper and lower premolars whereas later species have evolutionarily lost one premolar in each jaw. However, P. annectens has well-documented deciduous premolars, totaling at four in each of each first permanent molar before they are replaced by the three permanent premolars.[31] Remy argued that the first deciduous premolar was replaced by the first permanent premolar based on juvenille dentition of P. annectens, but Kenneth D. Rose et al. in 2017 argued that the demonstrated evidence did not prove Remy's hypothesis, meaning that it requires further research for proof. Most adult P. annectens individuals have no reported deciduous or permanent first premolars in either jaw, probably due to displacement by the second premolars.[43] The first premolar, when present, appears to be small, elongated, and narrow. The metacone cusp of P3 evolutionarily shrunk over time, and P4 at least sometimes lost its mesostyle cusp and often lost hypocone cusp. P4 has a high talonid cusp but lacks any entoconid cusp; the entoconid of P3 in comparison is short and a crescentlike shape. Within the molars, the ectoloph crest tends to stick out over the large cusps. The coronal cementum on the cheek teeth tend to thicken from the front end to the back end of the dental arch, and it tended to grow evolutionarily thicker over time. Paloplotherium sometimes lacks any coronal cementum. Within the upper molars, each ectoloph lobe has a middle rib developed on them. The paraconule cusp is separated from the protocone cusp, and the metaloph ridge only touches the ectoloph at advanced stages of dental wear. The crescents of the lower molars are separate from each other. Except for those in deciduous molars, the metastylid and metaconid cusps are nearly identical to each other. The internal cingulids of the lower molars are reduced or gone.[31]
Postcranial skeleton
[edit]
P. minor is known by a few incomplete skeletons, the first of which was studied originally by Georges Cuvier in 1804. According to Remy, the gypsum skeleton has been lost; he stated that the individual was a pregnant female. It was figured by Cuvier and later Blainville in 1839–1864, and the latter naturalist also figured skeletal elements from the French commune of Monthyon surrounding the skeleton whose whereabouts are also unclear.[31] P. minor is also known from another assembled skeleton that was originally documented by Fraas in the later 19th century,[12] although Stehlin referenced that Fraas paid little attention to studying the limb bones.[44] Remy in 2004 noted that the postcranial bones of palaeotheriids are not as well-studied, meaning that future studies would require studying traits of postcranial fossils of palaeotheres at the genus level.[31]
According to Remy, if the skeletal images as drawn by Cuvier and Blainville are accurate, then the back of P. minor appears convex, its peak being on par with the last thoracic vertebrae and its spinous processes of its lumbar vertebrae facing forward. Its arched back appears to be more similar to modern reconstructions of Propalaeotherium than to those of Palaeotherium. The cervical vertebrae of both Plagiolophus and Palaeotherium are elongated. It tail, composed of caudal vertebrae, has high spinous processes and appears pointed at its end. The tail is short in length and slender in spite of being made up of many vertebrae.[31]
Plagiolophus has several limb bone fossils attributed to it,[31] although it is unclear as to whether the tarsal and metatarsal bones from the Spanish locality of Roc de Santa are attributable to P. annectens or Anchilophus dumasi.[45][46] It is tridactyl, or three-toed, in its forelimbs and hindlimbs like most species of the fellow palaeotheriine Palaeotherium and unlike the earlier pachynolophine Propalaeotherium.[47] The scapula is forward-facing with a slightly narrow neck (its back being wider than its front) and a shortened upper edge. The iliac crest of the hip bone in Plagiolophus is concave in shape, contrasting with that of Palaeotherium which is convex. The foot bones of Plagiolophus are distinguished from those Palaeotherium based on its foot bones being more slender and its side toes being lesser-developed (or smaller and thinner) compared to its middle toe, suggesting that the digits are not well-supported anatomically. P. minor has particularly slender foot bones; the morphologies of the limb bones suggest that it was better-adapted to cursoriality than any species of Palaeotherium and other palaeothere genera.[31] The cursoriality adaptation in multiple Plagiolophus spp. along with Palaeotherium medium is supported by the elongated and gracile metacarpal bones which are of equal proportional lengths.[47] P. ministri has similarly tall and narrow astragali, suggesting that its limb bone morphologies could have been similar to those of P. minor. The astragali of P. huerzeleri are slightly shorter and wider compared to those of P. ministri. Both species have slender limb bones roughly corresponding to those of P. minor. P. fraasi differs from the aforementioned Plagiolophus species by the side metapodial bones being visible from the foot's front and the neck of the astragalus being visible. The astragali of P. javali and P. annectens are both short and stocky. The limb bone morphologies of P. annectens, P. fraasi, and P. javali point towards short and robust legs that were less adapted towards cursoriality.[31]
Footprints
[edit]Palaeotheriids are known from footprint tracks assigned to ichnotaxa, among them being the ichnogenus Plagiolophustipus, named in 1989–1990 by R. Santamaria et al. and suggested to have belonged to Plagiolophus. The ichonogenus is dated to the early Oligocene of Spain and may originated from the locality of Montfalco d'Agramunt, which the sole species name Plagiolophustipus montfalcoensis derives from. As a tridactyl footprint, it is diagnosed as having a middle digit that is much longer and wider than its two somewhat asymmetrical side digits. The ichonospecies measures between 5 cm (2.0 in) and 6 cm (2.4 in) total. The assignment of the ichnogenus to Plagiolophus is based on its middle digit being longer and wider than the other digits unlike that of Palaeotherium which has roughly equal sizes in all three of its toes.[48]
By extent, the ichnogenus Palaeotheriipus, assigned to Palaeotherium, differs from Plagiolophustipus by its smaller and wider digits. Lophiopus, likely produced by Lophiodon, has more divergent outer digit imprints, while Rhinoceripeda, attributed to the Rhinocerotidae, differs by its oval shape and varying from three to five digits.[49] Palaeotheriipus is known from both France and Iran whereas Plagiolophustipus is currently known from Spain.[50] It is possible that the ichnospecies is correlated with P. huerzeleri or another medium to large species based on their temporal ranges.[51]
Plagiolophustipus is also known by Plagiolophustipus ichsp. from the Spanish municipality of Mues in the province of Navarre, dating to the Oligocene. It is similar to Plagiolophustipus montfalcoensis because of the presence of three digits, the middle one of which is longer and wider than the other two side digits. The undefined ichnospecies could potentially have belonged a small to medium-sized palaeothere such as Plagiolophus.[52]
Size
[edit]
Plagiolophus is characterized by the inclusion of small to medium-sized species, the skull base length ranging from 140 mm (5.5 in) to 400 mm (16 in) depending on the species. The length of the P2 to M3 dental row ranges from 60 mm (2.4 in) to 121 mm (4.8 in). According to Remy, the basicranial (lower part of the skull) length of the Ma-PhQ-349 skull specimen of P. minor could have measured 176 mm (6.9 in) to 179 mm (7.0 in) long. Despite being a high, wide, and robust skull, P. minor is the smallest species of its genus, with the basal skull length being less than or equal to 200 mm (7.9 in) and the P2 to M3 dental row measuring 69 mm (2.7 in). P. huerzeleri is mentioned to have been 20-25% larger than P. ministri with a basicranial length of 350 mm (14 in) and a P2 to M3 dental row length of 100 mm (3.9 in) to 118 mm (4.6 in). The mandibular dental row of P. fraasi could measure 90 mm (3.5 in) to 98 mm (3.9 in) long whereas that of P. major could reach 99 mm (3.9 in) to 109 mm (4.3 in) long. The former species has an estimated skull length of 300 mm (12 in) while the latter's skull length could have measured 350 mm (14 in). P. javali is known only from a male juvenile mandible with a dental row measuring 121.2 mm (4.77 in) long. With a potential adult skull length of about 400 mm (16 in), P. javali is the largest species of Plagiolophus.[31]
Remy in 2004 calculated that the smallest species P. minor could have weighed less than 10 kg (22 lb). He also calculated P. huerzeleri to have a body weight range of 90 kg (200 lb) to 110 kg (240 lb). P. major has an estimated weight range of 90 kg (200 lb) to 110 kg (240 lb) while P. fraasi has an estimated weight range of 50 kg (110 lb) to 70 kg (150 lb). P. javali, as the largest species of Plagiolophus, could have had a body weight of over 150 kg (330 lb). [31] Later in 2015, he placed a body weight estimate of P. annectens at about 50 kg (110 lb), P. cartailhaci at 99 kg (218 lb), and P. mamertensis at 77 kg (170 lb).[39] Jamie A. MacLaren and Sandra Nauwelaerts in 2020 estimated the weight of P. minor at 19.3 kg (43 lb), P. annectens at 34.8 kg (77 lb), and P. major at 78.9 kg (174 lb).[47] In 2022, Leire Perales-Gogenola et al. made five weight estimates of different populations of Plagiolophus. They stated that P. mazateronensis from Mazaterón has a body weight of 118.71 kg (261.7 lb). According to the authors, P. minor from St. Capraise d'Eymet potentially weighed 26.56 kg (58.6 lb), and P. ministri from Villebramar weighed 53.61 kg (118.2 lb). They also said that P. annectens from Euzet weighed 34.8 kg (77 lb) while the same species from Roc de Santa I measured 40.6 kg (90 lb).[40] The same year, Perales-Gogenola et al. estimated that P. mazateronensis has a weight estimate range of 95 kg (209 lb) to 130 kg (290 lb).[42]
Palaeobiology
[edit]Plagiolophus contains several species of a wide range of sizes that are known from postcranial fossils that suggest different paces of locomotion, with some having bulky builds and some others being more cursorial.[31] Similar trends in limb morphological diversity and size diversity are also observed in a contemporary palaeothere Palaeotherium.[47] The evolutionary history of the palaeotheres might have had emphasized macrosmatic (derived smell) traits rather than sight or hearing, evident by the smaller orbits and a seeming lack of a derived auditory system. The macrosmatic trait could have allowed palaeotheres to keep track of their herds, implying gregarious behaviours. This is evident in Plagiolophus based on an implied development of the rhinencephalon, a portion of the brain concerning smell, in P. minor based on skull evidence.[53]
Both Palaeotherium and Plagiolophus have dentitions that are both capable of chewing through harder items such as fruits without wearing their teeth down quickly compared to their pachynolophine predecessors (i.e. Hyracotherium and Propalaeotherium). The shifts in dietary capabilities were the result of changes in the efficiencies of the mastication processes.[54] The broader diets of the later palaeotheres are the result of their molars serving dual purposes of shearing food on the buccal side then crushing it on the lingual side unlike in equids and basal equoids.[55] The two derived genera have brachyodont dentition, the hypsodonty index suggesting that both genera were mostly folivorous (leaf-eating) and did not have especially frugivorous (fruit-eating) tendencies because of the reduced proportions of rounded cusps. While both genera may have incorporated some fruit into their diets, the higher lingual tooth wear in Plagiolophus indicates it ate more fruit than Palaeotherium. Because of their likely tendencies to browse on higher plants, evident by their long necks and the woodland environments that they inhabited, it is unlikely that ground minerals, usually consumed from grazing on ground plants, significantly affected the tooth wear of either of the genera. The tooth wear in both genera could have been the result of scratches from chewing on fruit seeds. It is likely that Palaeotherium ate softer food such as younger leaves and fleshy fruit that may have had hard seeds while Plagiolophus leaned towards consuming tough food such as older leaves and harder fruit.[56] Palaeotherium consuming more leaf and woody material and less fruit compared to Plagiolophus is supported by the two having somewhat different chewing functions from each other and Palaeotherium having a high rate of efficiency in shearing food at lower energy.[55]
Similarly, Perales-Gogenola et al. observed that the Plagiolophus species that they studied all have brachyodont dentitions, but they also noted a general trend in hypsodonty within the genus over time. More specifically, they pointed out that early species tended to be very brachyodont but that later species tended to have more hypsodont dentition, potentially reaching a hypsodonty level similar to that in the Miocene North American endemic grazing equid Merychippus.[40] The hypsodonty trend in Plagiolophus was previously documented by Remy in 2004, who said that it is not known in Palaeotherium but that it was neither as rapid nor as dramatic a trend as in the hypsodonty observed in Leptolophus stehlini.[31] Leptolophus having a hypsodonty level similar to later Neogene equids suggests a distinct niche partitioning dietary strategy from contemporary palaeotheres, with Plagiolophus not showing a stricter preference towards abrasive plants based on dental evidence. Plagiolophus may have adopted dietary strategies similar to mixed-feeding deer such as the red deer (Cervus elephus) and roe deer (Capreolus capreolus) as well as the browsing-specialized moose (Alces alces), often avoiding hard foods (fruits, nuts, seeds, bark) and preferring tough leaves and related plant material. The changes in dietary behaviours in Plagiolophus were likely the result of environmental changes in western Europe during the late Eocene to early Oligocene.[40]
Both Plagiolophus and the anoplotheriid Diplobune consumed increasingly abrasive plant material during the Eocene-Oligocene transition, but Diplobune was purely a folivorous browser and therefore never consumed fruits unlike Plagiolophus.[57] The change in dieting in P. minor is evident from dental morphology and scratches in several localities of different time ranges. In the Late Eocene French locality of La Débruge (MP18), the cheek dentition of P. minor has a high amount of scratches resulting from wear created from the infrequent consumption of fruits and seeds, although its main diet consisted mainly of tough leaves. Its larger consumption of fruit is evident by the lower amount of round cusps and the few pits recorded in the teeth (the presence of more pits than scratches indicates more folivorous diets). In a later Late Eocene German locality of Frohnstetten (MP20) in comparison, the cheek teeth of P. minor have similar amount of pits but has more rounded cusps and slightly less scratches, suggesting that it consumed less fruit and more abrasive leaves. In Soumailles and Ronzon, both French localities dating after the Grande Coupure extinction event (MP21), the cheek teeth of P. minor has more rounded cusps, smaller pits, and more pits than scratches. The dental evidence likely implies that P. minor became a specialized browser to the extent that fruit is nearly absent from its diet. P. minor was also probably a less selective browser in the more easily available old and tough leaves that took more effort to consume, but it probably avoided younger leaves and shoots. The less specialized browsing diet could have been due to seasonal climates as well, in which the availability of certain plants by season varied. There are no significant changes in dental wear in P. minor from the older Soumailles locality to the younger Ronzon locality.[58]
Palaeoecology
[edit]Middle Eocene
[edit]
For much of the Eocene, a hothouse climate with humid, tropical environments with consistently high precipitations prevailed. Modern mammalian orders including the Perissodactyla, Artiodactyla, and Primates (or the suborder Euprimates) appeared already by the early Eocene, diversifying rapidly and developing dentitions specialized for folivory. The omnivorous forms mostly either switched to folivorous diets or went extinct by the middle Eocene (47–37 Ma) along with the archaic "condylarths". By the late Eocene (approx. 37–33 Ma), most of the ungulate form dentitions shifted from bunodont cusps to cutting ridges (i.e. lophs) for folivorous diets.[59][60]
Land-based connections to the north of the developing Atlantic Ocean were interrupted around 53 Ma, meaning that North America and Greenland were no longer well-connected to western Europe. From the early Eocene up until the Grande Coupure extinction event (56 Ma - 33.9 Ma), the western Eurasian continent was separated into three landmasses, the former two of which were isolated by seaways: western Europe (an archipelago), Balkanatolia, and eastern Eurasia (Balkanatolia was in between the Paratethys Sea of the north and the Neotethys Ocean of the south).[61] The Holarctic mammalian faunas of western Europe were therefore mostly isolated from other continents including Greenland, Africa, and eastern Eurasia, allowing for endemism to occur within western Europe.[60] The European mammals of the late Eocene (MP17 - MP20 of the Mammal Palaeogene zones) were mostly descendants of endemic middle Eocene groups as a result.[36]
In the fossil record, Plagiolophus is thought to have made its earliest appearance in MP12 based on dental fossils from the Geiseltal uMK locality in Germany that are classified as belonging to P. cartieri. The classification is typically only tentatively accepted by paleontologists due to the poor differentiation between Plagiolophus and Propalaeotherium in terms of the lower molars.[62][31][37] The earliest undisputed records of Plagiolophus are from the appearances of P. cartieri and P. casasecaensis in MP13 (the latter species of which is endemic to the Iberian peninsula and is restricted to the faunal unit).[40][31] By then, it would have coexisted with perissodactyls (Palaeotheriidae, Lophiodontidae, and Hyrachyidae), non-endemic artiodactyls (Dichobunidae and Tapirulidae), endemic European artiodactyls (Choeropotamidae (possibly polyphyletic, however), Cebochoeridae, and Anoplotheriidae), and primates (Adapidae). Both the Amphimerycidae and Xiphodontidae made their first appearances by the level MP14.[63][64] The stratigraphic ranges of the early species of Palaeotherium also overlapped with metatherians (Herpetotheriidae), cimolestans (Pantolestidae, Paroxyclaenidae), rodents (Ischyromyidae, Theridomyoidea, Gliridae), eulipotyphlans, bats, apatotherians, carnivoraformes (Miacidae), and hyaenodonts (Hyainailourinae, Proviverrinae).[65] Other MP13-MP14 sites have also yielded fossils of turtles and crocodylomorphs,[66] and MP13 sites are stratigraphically the latest to have yielded remains of the bird clades Gastornithidae and Palaeognathae.[67]
The Geiseltal Obere Mittelkhole locality, dating to MP13, records fossils of P. cartieri along with the herpetotheriid Amphiperatherium, carnivoraforme Quercygale, hyaenodont Proviverra, amphilemurid Amphilemur, archaeonycterid Matthesia, paroxyclaenid Pugiodens, adapid Europolemur, omomyid Nannopithex, dichobunid Messelobunodon, choeropotamids Rhagatherium and Amphirhagatherium, lophiodonts Lophiodon and Paralophiodon, and the other palaeotheres Propalaeotherium and Lophiotherium.[65]
The MP14 faunal unit marks the only known period in which P. lugdunensis appears and also records the final temporal appearance of P. cartieri. MP16 marks the first appearances of the species P. cartailhaci, P. curtisi, P. mamertensis, P. annectens, and P. mazateronensis, the former three of which were exclusive to the faunal unit. P. curtisi is known only from the United Kingdom, and P. mazateronensis was one of several palaeothere species endemic to the Iberian Peninsula. By the middle Eocene, Plagiolophus lived across western Europe in what is now Spain, the United Kingdom, France, Germany, and Switzerland.[31][36] [40] Despite being almost entirely recorded from western Europe, Plagiolophus sp. is recorded from an eastern European locality in Cherno More in Bulgaria, dating to the middle to late Eocene. The sporadic occurrences of Palaeotherium and Plagiolophus suggest some periodic connectivity between Balkanatolia and other Eurasian regions, allowing faunas to disperse between land.[68]
P. annectens, P. cartailhaci, and P. mamertensis are located in the MP16 French locality of Robiac along with the herpetotheriids Amphiperatherium and Peratherium, apatemyid Heterohyus, nyctithere Saturninia, omomyids (Necrolemur, Pseudoloris, and Microchoerus), adapid Adapis, ischyromyid Ailuravus, glirid Glamys, pseudosciurid Sciuroides, theridomyids Elfomys and Pseudoltinomys, hyaenodonts (Paracynohyaenodon, Paroxyaena, and Cynohyaenodon), carnivoraformes (Simamphicyon, Quercygale, and Paramiacis), cebochoerids Cebochoerus and Acotherulum, choeropotamids Choeropotamus and Haplobunodon, tapirulid Tapirulus, anoplotheriids (Dacrytherium, Catodontherium, and Robiatherium, dichobunid Mouillacitherium, robiacinid Robiacina, xiphodonts (Xiphodon, Dichodon, Haplomeryx), amphimerycid Pseudamphimeryx, lophiodont Lophiodon, hyrachyid Chasmotherium, and other palaeotheres (Palaeotherium, Leptolophus, Anchilophus, Metanchilophus, Lophiotherium, Pachynolophus, Eurohippus).[39]
After MP16, a faunal turnover occurred, marking the disappearances of the lophiodonts and European hyrachyids as well as the extinctions of all European crocodylomorphs except for the alligatoroid Diplocynodon.[64][66][69][70] The causes of the faunal turnover have been attributed to a shift from humid and highly tropical environments to drier and more temperate forests with open areas and more abrasive vegetation. The surviving herbivorous faunas shifted their dentitions and dietary strategies accordingly to adapt to abrasive and seasonal vegetation.[71][72] The environments were still subhumid and full of subtropical evergreen forests, however. The Palaeotheriidae was the sole remaining European perissodactyl group, and frugivorous-folivorous or purely folivorous artiodactyls became the dominant group in western Europe.[73][63]
Late Eocene
[edit]
The Late Eocene marks the latest appearances of P. annectens and P. mazateronensis at MP17 followed by the first appearances of P. oweni and P. minor at MP18 (the former of which is restricted to the unit). MP20 records both the continuous occurrence of P. minor and the restricted appearances of P. fraasi and P. major.[31] Within the late Eocene, the Cainotheriidae and derived members of the Anoplotheriinae both made their first fossil record appearances by MP18.[74][75] Also, several migrant mammal groups had reached western Europe by MP17a-MP18, namely the Anthracotheriidae, Hyaenodontinae, and Amphicyonidae.[65] In addition to snakes, frogs, and salamandrids, rich assemblage of lizards are known in western Europe as well from MP16-MP20, representing the Iguanidae, Lacertidae, Gekkonidae, Agamidae, Scincidae, Helodermatidae, and Varanoidea, most of which were able to thrive in the warm temperatures of western Europe.[76]
The MP18 locality of La Débruge in France holds fossil records of both P. oweni and P. minor along with the herpetotheriid Peratherium, theridomyids Blainvillimys and Theridomys, ischyromyid Plesiarctomys, glirid Glamys, hyaenodonts Hyaenodon and Pterodon, amphicyonid Cynodictis, palaeotheres Palaeotherium and Anchilophus, dichobunid Dichobune, choeropotamid Choeropotamus, cebochoerids Cebochoerus and Acotherulum, anoplotheriids (Anoplotherium, Diplobune, and Dacrytherium), tapirulid Tapirulus, xiphodonts Xiphodon and Dichodon, cainothere Oxacron, amphimerycid Amphimeryx, and the anthracothere Elomeryx.[65]
Grande Coupure
[edit]
The Grande Coupure event of western Europe is well-recognized in the palaeontological record as one of the largest extinction and faunal turnover events in the Cenozoic era.[77] The event is coincident with climate forcing events of cooler and more seasonal climates, the result being a 60% extinction rate of western European mammalian lineages while Asian faunal immigrants replaced them.[78][79][80] The Grande Coupure is often marked by palaeontologists as part of the Eocene-Oligocene boundary as a result at 33.9 Ma, although some estimate that the event began 33.6-33.4 Ma.[81][82] The event correlates directly with or after the Eocene-Oligocene transition, an abrupt shift from a greenhouse world characterizing much of the Paleogene to a coolhouse/icehouse world of the early Oligocene onwards. The massive drop in temperatures stems from the first major expansion of the Antarctic ice sheets that caused drastic pCO2 decreases and an estimated drop of ~70 m (230 ft) in sea level.[83]
The seaway dynamics separating western Europe from other landmasses to strong extents but allowing for some levels of dispersals prior to the Grande Coupure are complicated and contentious, but many palaeontologists agreed that glaciation and the resulting drops in sea level played major roles in the drying of the seaways previously acting as major barriers to eastern migrants from Balkanatolia and western Europe. The Turgai Strait is often proposed as the main European seaway barrier prior to the Grande Coupure, but some researchers challenged this perception recently, arguing that it completely receded already 37 Ma, long before the Eocene-Oligocene transition. Alexis Licht et al. suggested that the Grande Coupure could have possibly been synchronous with the Oi-1 glaciation (33.5 Ma), which records a decline in atmospheric CO2, boosting the Antarctic glaciation that already started by the Eocene-Oligocene transition. The Oi-1 glaciation, similar to the first glaciation event, caused large drops in sea level and pushed the global climate towards a coolhouse/icehouse environment.[61][84] The extinctions of a majority of endemic artiodactyls have been attributed to competition with immigrant faunas, environmental changes from cooling climates, or some combination of the two.[81]
The earliest Oligocene marked the arrivals of later anthracotheres, entelodonts, ruminants (Gelocidae, Lophiomerycidae), rhinocerotoids (Rhinocerotidae, Amynodontidae, Eggysodontidae), carnivorans (later Amphicyonidae, Amphicynodontidae, Nimravidae, and Ursidae), eastern Eurasian rodents (Eomyidae, Cricetidae, and Castoridae), and eulipotyphlans (Erinaceidae).[85][86][78][87]
In regard to palaeotheres, P. major and P. fraasi are recorded to have gone extinct by MP20 while P. minor survived past the Grande Coupure.[40] Research by Sarah C. Joomun et al. in 2010 suggests that P. minor changed its dietary habits most likely in response to increasingly abrasive plants, the result of environmental changes following the Oi-1 glaciation. Afterward, the climate in MP21 was stable enough that P. minor did not need to respond with further dietary changes.[58] The climatic trends from the Grande Coupure event favored palaeothere species that had light body builds and were built for cursoriality such as P. minor, allowing them to transverse across more open lands and escape from newly arrived predators where shelter otherwise was scarce.[47][53] P. fraasi in comparison had a stockier build,[31] a body build type that was likely unfavorable for early Oligocene environmental trends in relation to palaeotheres.[47][53]
In contrast to earlier faunal units, Plagiolophus in MP21 localities such as in Soumailles coexisted with both pre-Grande Coupure and immigrant faunas. P. minor was found in Soumailles along with the theridomyids Blainvillimys and Theridomys, nimravid Eusmilus, entelodont Entelodon, cebochoerid Acotherulum, eggysodont Eggysodon, and the palaeothere Palaeotherium.[65]
Early Oligocene
[edit]
Although the Eocene-Oligocene transition marked long-term drastic cooling global climates, western Eurasia was still dominated by humid climates, albeit with dry winter seasons in the Oligocene. Europe during the Oligocene had environments largely adapted to winter-dry seasons and humid seasons that were composed of three separate vegetational belts by latitude, with temperate needleleaf-broadleaved or purely broadleaved deciduous forests aligning with the northernmost belt between 40°N and 50°N, the middle belt of warmth-adapted mixed mesophytic and evergreen broadleaved forests aligning between 40°N and 30°N, and the last belt containing tropical vegetation aligning below 30°N.[88][89]
In the early Oligocene after MP21, Plagiolophus was the sole remaining palaeothere genus present in Europe.[90] P. minor is last recorded in MP22, but several other species are known to have originated during or after the Grande Coupure event. MP21 records the restricted temporal appearances of P. ovinus and P. ringeadei. Subsequent units contain one unique species of Plagiolophus: P. ministri in MP22, P. huerzeleri in MP23, and P. javali in MP25.[31] Several major faunal events occurred in the early Oligocene of Europe, namely the Bachitherium Dispersal Event in MP23 and a major faunal turnover event at MP24.[91][92]
In the MP25 French locality of Le Garouillas, the last surviving palaeothere species P. javali (the largest species of Plagiolophus) coexisted with the likes of the herpetotheriids Amphiperatherium and Peratherium, nyctithere Darbonetus, talpid Myxomygale, erinaceid Tetracus, bats (Vespertiliavus, Vaylatsia, Stehlinia), theridomyids (Blainvillimys, Issiodoromys, Theridomys), cricetid Eucricetodon, glirid Gliravus, nimravids (Quercylurus, Nimravus, Dinailurictis), amynodont Cadurcotherium, chalicothere Schizotherium, suoid Doliochoerus, dichobunid Metriotherium, cainotheres Plesiomeryx and Cainomeryx, lophiomerycid Lophiomeryx, and the bachithere Bachitherium.[65][31] MP25 corresponds to a period of high aridity in western Europe.[73]
See also
[edit]Notes
[edit]- ^ Due to archaic species naming conventions, authors of the 19th and 20th centuries tended to capitalize species names based on individuals or places.
References
[edit]- ^ Cuvier, Georges (1804). "Suite des Recherches: Suite de recherches sur les os fossiles de la pierre à plâtre des environs de Paris. Troisième mémoire. Restitution des pieds. Première section. Restitution des différens pieds de derrière". Annales du Muséum National d'Histoire Naturelle, Paris (in French). 3: 442–472. Archived from the original on 2023-07-27.
- ^ Cuvier, Georges (1804). "Suite de recherches sur les os fossiles de la pierre à plâtre des environs de Paris. Cinquiéme Mémoire. Sur les os du Tronc. Premiére Section. Description d'un squelette presque entier trouvé dans les carriéres de Pantin". Annales du Muséum National d'Histoire Naturelle, Paris (in French). 4: 66–81.
- ^ Cuvier, Geoges (1812). "Résumé général et rétablissement des Squelettes des diverses espèces". Recherches sur les ossemens fossiles de quadrupèdes: où l'on rétablit les caractères de plusieurs espèces d'animaux que les révolutions du globe paroissent avoir détruites (in French). Vol. 3. Chez Deterville. Archived from the original on 2023-07-31.
- ^ Rudwick, Martin J. S. (1997). "Chapter 6: The Animals from the Gypsum Beds around Paris". Georges Cuvier, Fossil Bones, and Geological Catastrophes: New Translations and Interpretations of the Primary Texts. University of Chicago Press.
- ^ a b Witton, Mark P.; Michel, Ellinor (2022). "Chapter 4: The sculptures: mammals". The Art and Science of the Crystal Palace Dinosaurs. The Crowood Press. pp. 68–91.
- ^ Aymard, Auguste (1846). "Essai monographie sur un nouveau genre de mammifère fossile trouvé dans la Haute-Loire, et nommé Entélodon". Annales de la Société d'Agriculture, Sciences, Arts et Commerce du Puy. 12: 227–267.
- ^ Pomel, Auguste (1847). "Note critique sur le genre Paléothérium". Bulletin de la Société géologique de France. 2. 4: 584–587.
- ^ a b Palmer, Theodore Sherman (1904). "A List of the Genera and Families of Mammals". North American Fauna (23). doi:10.3996/nafa.23.0001.
- ^ Owen, Richard (1848). "On the Fossil remains of Mammalia referable to the genus Palæotherium, and to two genera, Paloplotherium and Dichodon, hitherto undefined: from the Eocene Sand at Hordle, Hampshire". The Quarterly Journal of the Geological Society of London. 4 (1–2): 17. Bibcode:1848QJGS....4...17O. doi:10.1144/GSL.JGS.1848.004.01-02.11.
- ^ von Meyer, Christian Erich Hermann (1852). "Mitteilungen an Professor Bronn gerichtet". Neues Jahrbuch für Mineralogie, Geologie, Paläontologie, Stuttgart: 831–833.
- ^ Fraas, Oscar (1852). "Beitrage zu der Palaeotheriumformation". Jahreshefte des Vereins für vaterländische Naturkunde in Württemberg: 218–251.
- ^ a b Fraas, Oscar (1869). Die Geognostische Sammlung Wurttembergs: Im Erdgeschoss Des Koniglichen Naturalien-Cabinets Zu Stuttgart Ein Fuhrer Fur Die Besucher Derselben. Self-published.
- ^ Pomel, Auguste (1853). Catalogue méthodique et descriptif des Vertébrés fossiles découverts dans le bassin hydrographique supérieur de la Loir. J.B. Bailliere.
- ^ Ruetimeyer, Ludwig (1862). "Eocaene Säugethiere Gebiet des Schweizerischen Jura". Neue Denkschriften der Allg. Schweizerischen Gesellschaft für die Gesammten Naturwissenschaften. 19: 1–98.
- ^ Gaudry, Jean A. (1865). "Remarques sur les Paloplotherium". Nouvelles archives du Muséum d'histoire naturelle. 1: 15–24.
- ^ Pictet, François Jules; Humbert, Aloïs Humbert (1869). Mémoire sur les animaux vertébrés: trouvés dans le terrain sidérolitique du Canton de Vaud et appartenant a la faune éocène: supplément. Vol. 2. H. Georg.
- ^ Filhol, Henri (1877). "Recherches sur les phosphorites du Quercy: etude des fossiles qu'on y rencontre et spécialement des mammifères". Annales des sciences géologiques. 8: 160–167.
- ^ Rütimeyer, Ludwig (1891). "Die eocaene Säugethiere-Welt von Egerkingen. Gesammtdarstellung und dritter Nachtrag zu den "Eocänen Säugethieren aus dem Gebiet des schweizerischen Jura" (1862)". Abhandlungen der Schweizerischen paläontologischen Gesellschaft. 18: 61–62.
- ^ Depéret, Charles; Carrière, G. (1901). "Sur un nouveau gisement de mammifères de l'Eocène moyen à Robiac, près Saint-Mamert (Gard)". Comptes Rendus des séances de l'Académie des Sciences. 133: 616–618.
- ^ Dietrich, Wilhelm Otto (1922). "Beitrag zur Kenntnis der säugetierführenden Bohnerzformation in Schwaben. 1. Ein vergessenes, neu erschlossenes Höhlenvorkommen terrestrischen Eozäns auf der Ulmer Alb". Zentralblatt für Mineralogie, Geologie und Paläontologie. 19: 209–224.
- ^ Stehlin, Hans Georg (1904). "Sur les mammifères des Sables bartoniens du Castrais". Bulletin de la Société géologique de France. 4. 4: 445–475.
- ^ Stehlin, Hans Georg (1904). "Die Säugetiere des schweizerischen Eocaens. Critisher Catalog der Materialen. Zweiter Teil: Palaeotherium. — Plagiolophus. — Propalaeotherium". Abhandlungen der schweizerischen paläontologischen Gesellschaf. 31.
- ^ Depéret, Charles (1917). Monographie de la faune de mammifères fossiles du Ludien inférieur d'Euzet-les-Bains (Gard). Lyon A. Rey.
- ^ Remy, Jean Albert (1965). "Un nouveau genre de Paléothéridé (Perissodactyla) de l'Eocène supérieur du Midi de la France". Comptes Rendus de l'Académie des Sciences de Paris. 260: 4362–4364.
- ^ Hooker, Jerry J. (1986). "Mammals from the Bartonian (middle late Eocene) of the Hampshire Basin, southern England". Bulletin of the British Museum (Natural History) Geology. 39 (4): 191–478.
- ^ Brunet, Michel; Jehenne, Yves (1989). "Révision des genres Plagiolophus Pomel, 1847 et Paloplotherium Owen, 1848, Mammalia, Palaotheriidae du Paléogène d'Europe; intérêt biochronologique". Annales de Paléontologie. 75 (1): 23–52.
- ^ Remy, Jean A. (1994). "Une faunule de vertébrés sous la base des Grès de Célas (Eocène supérieur) à St-Dézéry (Gard)". Palaeovertebrata. 23 (1–4): 211–216.
- ^ a b c Ruiz-Colmenares, Miguel Ángel Cuesta (1994). "Los Plagiolophinae (Remy, 1976) nuevo rango (Perissodactyla, Mammalia) del Eoceno de la Cuenca del Duero (Castilla y Leon, España)". Estudios Geológicos. 50 (3–4): 253–279. doi:10.3989/egeol.94503-4323.
- ^ Soler, Lluís Checa (1997). "Los perisodàctilos (Mammalia, Ungulata) del eoceno catalàn". Paleontologia I Evolucio, Sabadell. 30–31: 149–234.
- ^ Remy, Jean A. (2000). "Plagiolophus huerzeleri, une nouvelle espèce de Palaeotheriidae (Perissodactyla, Mammalia) de l'Oligocène inférieur (Rupélien, MP 23), à Murs (Vaucluse, France)". Géobios. 33 (4): 489–503. Bibcode:2000Geobi..33..489R. doi:10.1016/S0016-6995(00)80082-0.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad Remy, Jean-Albert (2004). "Le genre Plagiolophus (Palaeotheriidae, Perissodactyla, Mammalia): révision systématique, morphologie et histologie dentaires, anatomie crânienne, essai d'interprétation fonctionnelle". Palaeovertebrata. 33 (1–4).
- ^ a b Bai, Bin (2017). "Eocene Pachynolophinae (Perissodactyla, Palaeotheriidae) from China, and their palaeobiogeographical implications". Palaeontology. 60 (6): 837–852. Bibcode:2017Palgy..60..837B. doi:10.1111/pala.12319.
- ^ a b Métais, Grégoire; Sen, Sevket (2017). "First occurrence of Palaeotheriidae (Perissodactyla) from the late–middle Eocene of eastern Thrace (Greece)". Comptes Rendus Palevol. 16 (4): 382–396. Bibcode:2017CRPal..16..382M. doi:10.1016/j.crpv.2017.01.001.
- ^ a b Bronnert, Constance; Métais, Grégoire (2023). "Early Eocene hippomorph perissodactyls (Mammalia) from the Paris Basin". Geodiversitas. 45 (9): 277–326. doi:10.5252/geodiversitas2023v45a9.
- ^ Bronnert, Constance; Gheerbrant, Emmanuel; Godinot, Marc; Métais, Grégoire (2017). "A primitive perissodactyl (Mammalia) from the early Eocene of Le Quesnoy (MP7, France)". Historical Biology. 30 (1–2): 237–250. doi:10.1080/08912963.2017.1341502.
- ^ a b c d Badiola, Ainara; Perales-Gogenola, Leire; Astibia, Humberto; Suberbiola, Xabier Pereda (2022). "A synthesis of Eocene equoids (Perissodactyla, Mammalia) from the Iberian Peninsula: new signs of endemism". Historical Biology. 34 (8): 1623–1631. Bibcode:2022HBio...34.1623B. doi:10.1080/08912963.2022.2060098. S2CID 248164842.
- ^ a b c Remy, Jean A.; Krasovec, Gabriel; Lopez, Éric; Marandat, Bernard; Lihoreau, Fabrice (2019). "The Palaeotheriidae (Equoidea, Perissodactyla, Mammalia) from the Eocene fauna of Aumelas (Hérault department, France)". Geodiversitas. 41 (1): 525–585. doi:10.5252/geodiversitas2019v41a13.
- ^ a b Remy, Jean A. (2017). "Critical comments on the genus Propachynolophus Lemoine, 1891 (Mammalia, Perissodactyla, Equoidea)". Palaeovertebrata. 41: 1–18. doi:10.18563/pv.41.1.e3.
- ^ a b c Remy, Jean-Albert (2015). "Les Périssodactyles (Mammalia) du gisement Bartonien supérieur de Robiac (Éocène moyen du Gard, Sud de la France)". Palaeovertebrata. 39 (1): 1–98. doi:10.18563/pv.39.1.e3.
- ^ a b c d e f g h Perales-Gogenola, Leire; Merceron, Gildas; Badiola, Ainara; Gómez-Olivencia, Asier; Suberbiola, Xabier Pereda (2022). "The evolutionary ecology of the endemic European Eocene Plagiolophus (Mammalia: Perissodactyla)". Palaeogeography, Palaeoclimatology, Palaeoecology. 594. Bibcode:2022PPP...59410962P. doi:10.1016/j.palaeo.2022.110962. hdl:10810/61572.
- ^ a b Franzen, Jens L. (1968). Revision der Gattung Palaeotherium Cuvier, 1804 (Palaeotheriidae, Perissodactyla, Mammalia). Band 1 (Inaugural Dissertation). University of Freiburg.
- ^ a b Perales-Gogenola, Leire; Badiola, Ainara; Pereda-Suberbiola, Xabier; Astibia, Humberto (2022). "New Eocene fossil remains of Palaeotheriidae (Perissodactyla, Mammalia) from Mazaterón (Soria, Castile and Leon, Spain)". Historical Biology. 34 (8): 1388–1398. Bibcode:2022HBio...34.1388P. doi:10.1080/08912963.2021.2025363.
- ^ Rose, Kenneth D.; Holbrook, Luke T.; Luckett, Patrick (2018). "Deciduous premolars of Eocene Equidae and their phylogenetic significance". Historical Biology. 30 (1–2): 89–118. Bibcode:2018HBio...30...89R. doi:10.1080/08912963.2017.1291637.
- ^ Stehlin, Hans Georg (1938). "Zur Charakteristik einiger Palaeotheriumarten des oberen Ludien". Eclogae Geologicae Helvetiae. 31 (2): 263–292.
- ^ Casanovas-Cladellas, María Lourdes; Santafé Llopis, José Vicente (1981). "Descripcion de elementos tarsales y metatarsales de Plagiolophus annectens y Anchilophus dumasi (Palaeotheriidae, Perissodactyla) del yacimiento de Roc de Santa (Area del Noguera Pallaresa)". Paleontologia i Evolució. 16: 29–37.
- ^ Casanovas-Cladellas, María Lourdes; Checa-Soler, Lluis; Santafé Llopis, José Vicente (1993). "Esqueleto postcraneal de los Equoidea de talla media del yacimiento Ludiense de Roc de Santa (área del Noguera Pallaresa, Lleida, España)". Revista Española de Paleontología. 8 (1): 37–55.
- ^ a b c d e f MacLaren, Jamie A.; Nauwelaerts, Sandra (2020). "Modern tapirs as morphofunctional analogues for locomotion in endemic Eocene European perissodactyls". Journal of Mammalian Evolution. 27 (2): 245–263. doi:10.1007/s10914-019-09460-1.
- ^ Santamaria, R.; Gregorio, López; Casanovas-Cladellas, María Lourdes (1989–1990). "Nuevos yacimientos con icnitas de mamíferos del Oligoceno de los alrededores de Agramunt (Lleida, España)". Paleontologia i Evolució (23): 141–152.
- ^ Abbassi, Nasrollah; Lucas, Spencer G.; Gholam, Reza Zaare (2015). "First report of Oligocene vertebrate footprints from Iran". Palaeogeography, Palaeoclimatology, Palaeoecology. 440: 78–89. Bibcode:2015PPP...440...78A. doi:10.1016/j.palaeo.2015.08.039.
- ^ Belvedere, Matteo; Fabre, Emmanuel; Pandolfi, Luca; Legal, Stephane; Coster, Pauline (2023). "Stepping into Oligocene. A reassessment of the early Oligocene mammal tracks from Saignon (SE France)". Historical Biology: 1–17. doi:10.1080/08912963.2023.2286275.
- ^ Montes, Martín Linares; Luzón, Aránzazu; Cuenca-Bescós, Gloria; Canudo, José Ignacio; Castanera, Diego (2022). "New mammal and bird tracks from the Lower Oligocene of the Ebro Basin (NE Spain): implications for the Palaeogene ichnological record". Historical Biology. 35 (9): 1616–1636. doi:10.1080/08912963.2022.2104644.
- ^ Murelaga, Xabier; Baceta, Juan Ignacio; Astibia, Humberto; Badiola, Ainara (1999). "Icnitas de perisodáctilos en el Oligoceno de Navarra: posición estratigráfica y sistemática". Geogaceta. 27: 15–18.
- ^ a b c Santi, Guiseppe (2000). "Palaeotheriidae (Perissodactyla, Mammalia) Del Paleogene Dell'Europa Centrale: Note E Considerazioni Preliminari". Natura Bresciana. 32: 15–22.
- ^ Engels, Sandra (2010). Functional and morphological changes in the molar morphology of early Hippomorpha (PDF). Jahrestagung der Paläontologischen Gesellschaft 2010. pp. 33–34.
- ^ a b Engels, Sandra; Schultz, Julia A. (2018). "Evolution of the power stroke in early Equoidea (Perissodactyla, Mammalia)". Palaeobiodiversity and Palaeoenvironments. 99 (2): 271–291. Bibcode:2019PdPe...99..271E. doi:10.1007/s12549-018-0341-4.
- ^ Joomun, Sarah C.; Hooker, Jerry J.; Collinson, Margaret E. (2008). "Dental wear variation and implications for diet: An example from Eocene perissodactyls (Mammalia)". Palaeogeography, Palaeoclimatology, Palaeoecology. 263 (3–4): 92–106. Bibcode:2008PPP...263...92J. doi:10.1016/j.palaeo.2008.03.001.
- ^ Joomun, Sarah C.; Hooker, Jerry J.; Collinson, Margaret E. (2009). Differences in the Dietary Responses of the Perissodactyl Plagiolophus and the Artiodactyl Diplobune to the Eocene/Oligocene Transition Events in Europe (PDF). 69th Annual Meeting Society of Vertebrate Paleontology and the 57th Symposium of Vertebrate Palaeontology and Comparative Anatomy (SVPCA). Vol. 29.
- ^ a b Joomun, Sarah C.; Hooker, Jerry J.; Collins on, Margaret E. (2010). "Changes in Dental Wear of Plagiolophus minor (Mammalia: Perissodactyla) Across the Eocene—Oligocene Transition". Journal of Vertebrate Paleontology. 30 (2): 563–576. Bibcode:2010JVPal..30..563J. doi:10.1080/02724631003618124.
- ^ Eronen, Jussi T.; Janis, Christine M.; Chamberlain, Charles Page; Mulch, Andreas (2015). "Mountain uplift explains differences in Palaeogene patterns of mammalian evolution and extinction between North America and Europe". Proceedings of the Royal Society B: Biological Sciences. 282 (1809): 20150136. doi:10.1098/rspb.2015.0136. PMC 4590438. PMID 26041349.
- ^ a b Maitre, Elodie (2014). "Western European middle Eocene to early Oligocene Chiroptera: systematics, phylogeny and palaeoecology based on new material from the Quercy (France)". Swiss Journal of Palaeontology. 133 (2): 141–242. Bibcode:2014SwJP..133..141M. doi:10.1007/s13358-014-0069-3. S2CID 84066785.
- ^ a b Licht, Alexis; Métais, Grégoire; Coster, Pauline; İbilioğlu, Deniz; Ocakoğlu, Faruk; Westerweel, Jan; Mueller, Megan; Campbell, Clay; Mattingly, Spencer; Wood, Melissa C.; Beard, K. Christopher (2022). "Balkanatolia: The insular mammalian biogeographic province that partly paved the way to the Grande Coupure". Earth-Science Reviews. 226: 103929. Bibcode:2022ESRv..22603929L. doi:10.1016/j.earscirev.2022.103929.
- ^ Hellmund, Meinolf (2000). "Erstnachweis von Plagiolophus cartieri Stehlin (Palaeotheriidae, Perissodactyla) in der unteren Mittelkohle (uMK, MP 12) des Geiseltales bei Halle (Sachsen-Anhalt, Deutschland)". Neues Jahrbuch für Mineralogie Geologie Paläontologie. 4: 205–216. doi:10.1127/njgpm/2000/2000/205.
- ^ a b Blondel, Cécile (2001). "The Eocene-Oligocene ungulates from Western Europe and their environment" (PDF). Palaeogeography, Palaeoclimatology, Palaeoecology. 168 (1–2): 125–139. Bibcode:2001PPP...168..125B. doi:10.1016/S0031-0182(00)00252-2.
- ^ a b Franzen, Jens Lorenz (2003). "Mammalian faunal turnover in the Eocene of central Europe". Geological Society of America Special Papers. 369: 455–461. doi:10.1130/0-8137-2369-8.455. ISBN 9780813723693.
- ^ a b c d e f Aguilar, Jean-Pierre; Legendre, Serge; Michaux, Jacques (1997). "Synthèses et tableaux de corrélations". Actes du Congrès Bio-chroM'97. Mémoires et Travaux de l'EPHE Institut de Montpellier 21 (in French). École Pratique des Hautes Études-Sciences de la Vie et de la Terre, Montpellier. pp. 769–850.
- ^ a b Martin, Jeremy E.; Pochat-Cottilloux, Yohan; Laurent, Yves; Perrier, Vincent; Robert, Emmanuel; Antoine, Pierre-Olivier (2022). "Anatomy and phylogeny of an exceptionally large sebecid (Crocodylomorpha) from the middle Eocene of southern France". Journal of Vertebrate Paleontology. 42 (4). Bibcode:2022JVPal..42E3828M. doi:10.1080/02724634.2023.2193828. S2CID 258361595.
- ^ Buffetaut, Eric; Angst, Delphine (2014). Stratigraphic Distribution of Large Flightless Birds in the Palaeogene of Europe. STRATI 2013: First International Congress on Stratigraphy At the Cutting Edge of Stratigraphy. doi:10.1007/978-3-319-04364-7_190.
- ^ Métais, Grégoire; Coster, Pauline; Kaya, Mustafa; Licht, Alexis; Miller, Kristen; Ocakoğlu, Faruk; Rust, Kathleen; Beard, K. Christopher (2024). "Rapid colonization and diversification of a large-bodied mammalian herbivore clade in an insular context: New embrithopods from the Eocene of Balkanatolia". Journal of Mammalian Evolution. 31 (15). doi:10.1007/s10914-024-09711-w.
- ^ Martin, Jeremy E. (2015). "A sebecosuchian in a middle Eocene karst with comments on the dorsal shield in Crocodylomorpha". Acta Palaeontologica Polonica. 60 (3): 673–680. doi:10.4202/app.00072.2014. S2CID 54002673.
- ^ Antunes, Miguel Telles (2003). "Lower Paleogene Crocodilians from Silveirinha, Portugal". Palaeovertebrata. 32: 1–26.
- ^ Robinet, Céline; Remy, Jean Albert; Laurent, Yves; Danilo, Laure; Lihoreau, Fabrice (2015). "A new genus of Lophiodontidae (Perissodactyla, Mammalia) from the early Eocene of La Borie (Southern France) and the origin of the genus Lophiodon Cuvier, 1822". Geobios. 48 (1): 25–38. Bibcode:2015Geobi..48...25R. doi:10.1016/j.geobios.2014.11.003.
- ^ Perales-Gogenola, Leire; Badiola, Ainara; Gómez-Olivencia, Asier; Pereda-Suberbiola, Xabier (2022). "A remarkable new paleotheriid (Mammalia) in the endemic Iberian Eocene perissodactyl fauna". Journal of Vertebrate Paleontology. 42 (4). Bibcode:2022JVPal..42E9447P. doi:10.1080/02724634.2023.2189447. S2CID 258663753.
- ^ a b Solé, Floréal; Fischer, Valentin; Le Verger, Kévin; Mennecart, Bastien; Speijer, Robert P.; Peigné, Stéphane; Smith, Thierry (2022). "Evolution of European carnivorous mammal assemblages through the Paleogene". Biological Journal of the Linnean Society. 135 (4): 734–753. doi:10.1093/biolinnean/blac002.
- ^ Erfurt, Jörg; Métais, Grégoire (2007). "Endemic European Paleogene Artiodactyls". In Prothero, Donald R.; Foss, Scott E. (eds.). The Evolution of Artiodactyls. Johns Hopkins University Press. pp. 59–84.
- ^ Weppe, Romain; Blondel, Cécile; Vianey-Liaud, Monique; Escarguel, Gilles; Pelissie, Thierry; Antoine, Pierre-Olivier; Orliac, Maeva J. (2020). "Cainotheriidae (Mammalia, Artiodactyla) from Dams (Quercy, SW France): phylogenetic relationships and evolution around the Eocene–Oligocene transition (MP19–MP21)" (PDF). Journal of Systematic Palaeontology. 18 (7): 541–572. Bibcode:2020JSPal..18..541W. doi:10.1080/14772019.2019.1645754. S2CID 202026238.
- ^ Rage, Jean-Claude (2012). "Amphibians and squamates in the Eocene of Europe: what do they tell us?". Palaeobiodiversity and Palaeoenvironments. 92 (4): 445–457. Bibcode:2012PdPe...92..445R. doi:10.1007/s12549-012-0087-3. S2CID 128651937.
- ^ Sun, Jimin; Ni, Xijun; Bi, Shundong; Wu, Wenyu; Ye, Jie; Meng, Jin; Windley, Brian F. (2014). "Synchronous turnover of flora, fauna, and climate at the Eocene-Oligocene Boundary in Asia". Scientific Reports. 4 (7463): 7463. Bibcode:2014NatSR...4.7463S. doi:10.1038/srep07463. PMC 4264005. PMID 25501388.
- ^ a b Hooker, Jerry J.; Collinson, Margaret E.; Sille, Nicholas P. (2004). "Eocene–Oligocene mammalian faunal turnover in the Hampshire Basin, UK: calibration to the global time scale and the major cooling event" (PDF). Journal of the Geological Society. 161 (2): 161–172. Bibcode:2004JGSoc.161..161H. doi:10.1144/0016-764903-091. S2CID 140576090.
- ^ Legendre, Serge; Mourer-Chauviré, Cécile; Hugueney, Marguerite; Maitre, Elodie; Sigé, Bernard; Escarguel, Gilles (2006). "Dynamique de la diversité des mammifères et des oiseaux paléogènes du Massif Central (Quercy et Limagnes, France)". STRATA. 1 (in French). 13: 275–282.
- ^ Escarguel, Gilles; Legendre, Serge; Sigé, Bernard (2008). "Unearthing deep-time biodiversity changes: The Palaeogene mammalian metacommunity of the Quercy and Limagne area (Massif Central, France)". Comptes Rendus Geoscience. 340 (9–10): 602–614. Bibcode:2008CRGeo.340..602E. doi:10.1016/j.crte.2007.11.005.
- ^ a b Costa, Elisenda; Garcés, Miguel; Sáez, Alberto; Cabrera, Lluís; López-Blanco, Miguel (2011). "The age of the "Grande Coupure" mammal turnover: New constraints from the Eocene–Oligocene record of the Eastern Ebro Basin (NE Spain)". Palaeogeography, Palaeoclimatology, Palaeoecology. 301 (1–4): 97–107. Bibcode:2011PPP...301...97C. doi:10.1016/j.palaeo.2011.01.005. hdl:2445/34510.
- ^ Hutchinson, David K.; Coxall, Helen K.; Lunt, Daniel J.; Steinthorsdottir, Margret; De Boer, Agatha M.; Baatsen, Michiel L.J.; Von der Heydt, Anna S.; Huber, Matthew; Kennedy-Asser, Alan T.; Kunzmann, Lutz; Ladant, Jean-Baptiste; Lear, Caroline; Moraweck, Karolin; Pearson, Paul; Piga, Emanuela; Pound, Matthew J.; Salzmann, Ulrich; Scher, Howie D.; Sijp, Willem P.; Śliwińska, Kasia K; Wilson, Paul A.; Zhang, Zhongshi (2021). "The Eocene-Oligocene transition: A review of marine and terrestrial proxy data, models and model-data comparisons". Climate of the Past. 17 (1): 269–315. Bibcode:2021CliPa..17..269H. doi:10.5194/cp-17-269-2021. S2CID 234099337.
- ^ Toumoulin, Agathe; Tardif, Delphine; Donnadieu, Yannick; Licht, Alexis; Ladant, Jean-Baptiste; Kunzmann, Lutz; Dupont-Nivet, Guillaume (2022). "Evolution of continental temperature seasonality from the Eocene greenhouse to the Oligocene icehouse –a model–data comparison". Climate of the Past. 18 (2): 341–362. Bibcode:2022CliPa..18..341T. doi:10.5194/cp-18-341-2022.
- ^ Boulila, Slah; Dupont-Nivet, Guillaume; Galbrun, Bruno; Bauer, Hugues; Châteauneuf, Jean-Jacques (2021). "Age and driving mechanisms of the Eocene–Oligocene transition from astronomical tuning of a lacustrine record (Rennes Basin, France)". Climate of the Past. 17 (6): 2343–2360. Bibcode:2021CliPa..17.2343B. doi:10.5194/cp-17-2343-2021. S2CID 244097729.
- ^ Rivals, Florent; Belyaev, Ruslan I.; Basova, Vera B.; Prilepskaya, Natalya E. (2023). "Hogs, hippos or bears? Paleodiet of European Oligocene anthracotheres and entelodonts". Palaeogeography, Palaeoclimatology, Palaeoecology. 611: 111363. Bibcode:2023PPP...61111363R. doi:10.1016/j.palaeo.2022.111363. S2CID 254801829.
- ^ Becker, Damien (2009). "Earliest record of rhinocerotoids (Mammalia: Perissodactyla) from Switzerland: systematics and biostratigraphy". Swiss Journal of Geosciences. 102 (3): 489–504. Bibcode:2009SwJG..102..489B. doi:10.1007/s00015-009-1330-4. S2CID 67817430.
- ^ Solé, Floréal; Fischer, Fischer; Denayer, Julien; Speijer, Robert P.; Fournier, Morgane; Le Verger, Kévin; Ladevèze, Sandrine; Folie, Annelise; Smith, Thierry (2020). "The upper Eocene-Oligocene carnivorous mammals from the Quercy Phosphorites (France) housed in Belgian collections". Geologica Belgica. 24 (1–2): 1–16. doi:10.20341/gb.2020.006. S2CID 224860287.
- ^ Utescher, Torsten; Erdei, Boglárka; François, Louis; Henrot, Alexandra-Jane; Mosbrugger, Volker; Popova, Svetlana (2020). "Oligocene vegetation of Europe and western Asia-Diversity change and continental patterns reflected by plant functional types". Geological Journal. 56 (2): 628–649. doi:10.1002/gj.3830. S2CID 216198894.
- ^ Moreno-Domínguez, Rafael; Postigo-Mijarra, José Mª.; Barrón, Eduardo (2021). "Palaeoclimatic reconstruction for the Late Oligocene La Val fossil site (Estadilla, Huesca, Spain) based on CLAMP and LMA". Palaeogeography, Palaeoclimatology, Palaeoecology. 567 (3): 110302. Bibcode:2021PPP...56710302M. doi:10.1016/j.palaeo.2021.110302. S2CID 233968947.
- ^ Scherler, Laureline; Mennecart, Bastien; Hiard, Florent; Becker, Damien (2013). "Evolutionary history of hoofed mammals during the Oligocene–Miocene transition in Western Europe". Swiss Journal of Geosciences. 106 (2): 349–369. Bibcode:2013SwJG..106..349S. doi:10.1007/s00015-013-0140-x. S2CID 128585686.
- ^ Mennecart, Bastien; Aiglstorfer, Manuela; Li, Yikun; Li, Chunxiao; Wang, Shiqi (2021). "Ruminants reveal Eocene Asiatic palaeobiogeographical provinces as the origin of diachronous mammalian Oligocene dispersals into Europe". Scientific Reports. 11 (17710): 17710. Bibcode:2021NatSR..1117710M. doi:10.1038/s41598-021-96221-x. PMC 8421421. PMID 34489502.
- ^ Mennecart, Bastien; Métais, Grégoire (2015). "Mosaicomeryx gen. nov., a ruminant mammal from the Oligocene of Europe and the significance of 'gelocids'". Journal of Systematic Palaeontology. 13 (7): 581–600. Bibcode:2015JSPal..13..581M. doi:10.1080/14772019.2014.948505. S2CID 56365084.