Organoantimony chemistry
Organoantimony chemistry is the chemistry of compounds containing a carbon to antimony (Sb) chemical bond. Relevant oxidation states are SbV and SbIII. The toxicity of antimony[1] limits practical application in organic chemistry.[2]
Syntheses
[edit]Stibines
[edit]An organoantimony synthesis typically begins with tricoordinate antimony compounds, called stibines. Antimony trichloride reacts with organolithium or Grignard reagents to give compounds of the form R3Sb:
- SbCl3 + 3 RLi (or RMgCl) → R3Sb
Stibines are weak Lewis acids and do not form ate complexes. As soft Lewis donors, they see wide use in coordination chemistry[3]: 348 and typically react through oxidative addition:
- R3Sb + Br2 → R3SbBr2
- R3Sb + O2 → R3SbO
- R3Sb + B2H6 → R3Sb·BH3
This property also sensitizes them to air.
If reduced instead, stibanes typically release substituents (ligands):[3]: 443
- R3Sb + Na + NH3 → R2SbNa
- R2SbBr + Mg → (R2Sb)2 + MgBr2
The cyclic compound stibole, a structural analog of pyrrole, has not been isolated, but substituted derivatives have. Antimony metallocenes are known as well:
- 14SbI3 + 3 (Cp*Al)4 → [Cp∗
2Sb]+[AlI4]− + 8Sb + 6 AlI3
The Cp*-Sb-Cp* angle is 154°.
Stiboranes
[edit]Pentacoordinate antimony compounds are called stiboranes, and can be synthesised from stibines and halogens:
Like their heavier congeners, the organobismuth compounds, stiboranes form onium compounds and ate complexes. Asymmetric compounds can also be obtained through the stibonium ion:
- R5Sb + X2 → [R4Sb]+[X]
- [R4Sb]+[X] + R'MgX → R4R'Sb
Stibonium halides (R4SbX) tend to dimerize.
Trigonal-bipyramidal molecule pentaphenylantimony decomposes at 200 °C to triphenylstibine and biphenyl. In the related Me5Sb, proton NMR at -100 °C cannot resolve different methyl protons.
Distibines and antimony(I) compounds
[edit]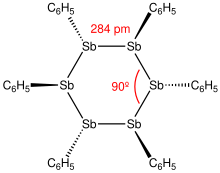
Distibines are formally SbII compounds, but feature tricoordinate Sb atoms with a single Sb-Sb bond. They may have interest as thermochromes. For example, tetramethyldistibine is colorless when gas, yellow when liquid, red when solid just below the melting point of 18.5 °C, shiny-blue when cooler, and again yellow at cryogenic temperatures.[5][3]: 442 A typical synthesis first displaces an SbIII halide with an alkali metal and then reduces the resulting anion with ethylene dichloride.[3]: 781–783
Like its lighter congener, arsenic, organoantimony compounds can be reduced to cyclic oligomers that are formally antimony(I) compounds.[3]: 563–577
With other substituents
[edit]SbV-N bonds are unstable, except where the N is also bonded to other electron-withdrawing substituents.[6]
Reactions
[edit]Stibine oxides undergo a sort of polarized-olefin metathesis. For example, they mediate a carbonyl-imine exchange (Ar is any activated arene):[7]: 399
Ph3Sb=NSO2Ar + PhC=O → Ph3Sb=O + PhC=NSO2Ar
The effect may extend vinylically:[8] In contrast, unstabilized ylides (R3Sb=CR'2; R' not electron-withdrawing) form only with difficulty (e.g. diazo reagents).[7]: 399–400
Like other metals, stibanes vicinal to a leaving group can eliminate before a proton. For example, diphenyl(β-hydroxyphenethyl)stibine decomposes in heat or acid to styrene:[7]: 400–402
- Ph2SbCH2CH(OH)Ph → CH2=CHPh + Ph2SbOH
As tertiary stibines also insert into haloalkyl bonds, tertiary stibines are powerful dehalogenating agents.[7]: 403 However, stibanes poorly imitate active metal organometallics: only with difficulty do their ligands add to carbonyls or they power noble-metal cross couplings.[7]: 403–405
Stiboranes are gentle oxidants, converting acyloins to diketones and thiols to disulfides.[7]: 406–408 In air, tris(thiophenyl)stibine catalyzes a Hunsdiecker-like decarboxylative oxidation of anhydrides to alcohols.[7]: 411
In ultraviolet light, distibines radicalize; the resulting radicals can displace iodide.[3]: 766
See also
[edit]References
[edit]- ^ Filella, M. (2010). "Alkyl derivatives of antimony in the environment". Metal Ions in Life Sciences. 7. Cambridge: RSC publishing: 267–301. doi:10.1039/9781849730822-00267. ISBN 978-1-84755-177-1. PMID 20877810.
- ^ C. Elschenbroich, A. Salzer Organometallics : A Concise Introduction (2nd Ed) (1992) from Wiley-VCH: Weinheim. ISBN 3-527-28165-7
- ^ a b c d e f Patai, Saul, ed. (1994). The Chemistry of Organic Arsenic, Antimony, and Bismuth Compounds. Chemistry of Functional Groups. Chichester, UK: Wiley. doi:10.1002/0470023473. ISBN 047193044X.
- ^ . doi:10.1002/anie.198500721.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ Organoantimony compounds with element-element bonds H.J. Breunig, R. Rosler Coordination Chemistry Reviews 163 (1997) 33-53
- ^ Patai 1994, p. 340, which immediately undercuts itself by giving an example of an -SbCl3-NMe-... complex.
- ^ a b c d e f g Freedman, Leon D.; Doak, George O. (1989). "The use of organoantimony and organobismuth compounds in organic synthesis". In Hartley, Frank Robinson (ed.). The Chemistry of the Metal—Carbon Bond. (Patai's) Chemistry of Functional Groups. Vol. 5. Chichester, UK: Interscience. pp. 397–413. doi:10.1002/9780470772263.ch9. ISBN 0471915564.
- ^ Freedman & Doak 1989, p. 410, which ascribes the reaction instead to a Wittig-type reaction with an ylide.

![{\displaystyle {\ce {R2C=O{}+HBrCHCO2R->[{} \atop {\ce {Bu3Sb}}]R2C=CHCO2R{}+HBr}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/822cb5341df8cb99cdcf16f281181770d3db98a9)