Multiomics
You can help expand this article with text translated from the corresponding article in French. (August 2021) Click [show] for important translation instructions.
|
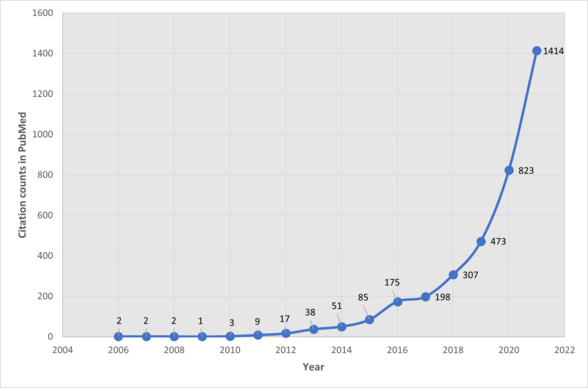
Multiomics, multi-omics, integrative omics, "panomics" or "pan-omics" is a biological analysis approach in which the data sets are multiple "omes", such as the genome, proteome, transcriptome, epigenome, metabolome, and microbiome (i.e., a meta-genome and/or meta-transcriptome, depending upon how it is sequenced);[1][2][3] in other words, the use of multiple omics technologies to study life in a concerted way. By combining these "omes", scientists can analyze complex biological big data to find novel associations between biological entities, pinpoint relevant biomarkers and build elaborate markers of disease and physiology. In doing so, multiomics integrates diverse omics data to find a coherently matching geno-pheno-envirotype relationship or association.[4] The OmicTools service lists more than 99 pieces of software related to multiomic data analysis, as well as more than 99 databases on the topic.
Systems biology approaches are often based upon the use of panomic analysis data.[5][6] The American Society of Clinical Oncology (ASCO) defines panomics as referring to "the interaction of all biological functions within a cell and with other body functions, combining data collected by targeted tests ... and global assays (such as genome sequencing) with other patient-specific information."[7]
Single-cell multiomics
[edit]A branch of the field of multiomics is the analysis of multilevel single-cell data, called single-cell multiomics.[8][9] This approach gives us an unprecedent resolution to look at multilevel transitions in health and disease at the single cell level. An advantage in relation to bulk analysis is to mitigate confounding factors derived from cell to cell variation, allowing the uncovering of heterogeneous tissue architectures.[8]
Methods for parallel single-cell genomic and transcriptomic analysis can be based on simultaneous amplification[10] or physical separation of RNA and genomic DNA.[11] They allow insights that cannot be gathered solely from transcriptomic analysis, as RNA data do not contain non-coding genomic regions and information regarding copy-number variation, for example. An extension of this methodology is the integration of single-cell transcriptomes to single-cell methylomes, combining single-cell bisulfite sequencing[12][13] to single cell RNA-Seq.[14] Other techniques to query the epigenome, as single-cell ATAC-Seq[15] and single-cell Hi-C[16] also exist.
A different, but related, challenge is the integration of proteomic and transcriptomic data.[17][18] One approach to perform such measurement is to physically separate single-cell lysates in two, processing half for RNA, and half for proteins.[17] The protein content of lysates can be measured by proximity extension assays (PEA), for example, which use DNA-barcoded antibodies.[19] A different approach uses a combination of heavy-metal RNA probes and protein antibodies to adapt mass cytometry for multiomic analysis.[18]
Related to Single-cell multiomics is the field of Spatial Omics which assays tissues through omics readouts that preserve the relative spatial orientation of the cells in the tissue. The number of Spatial Omics methods published still lags behind the number of methods published for Single-Cell multiomics, but the numbers are catching up (Single-cell and Spatial methods).
Multiomics and machine learning
[edit]In parallel to the advances in high-throughput biology, machine learning applications to biomedical data analysis are flourishing. The integration of multi-omics data analysis and machine learning has led to the discovery of new biomarkers.[20][21][22] For example, one of the methods of the mixOmics project implements a method based on sparse Partial Least Squares regression for selection of features (putative biomarkers).[23] A unified and flexible statistical framewok for heterogeneous data integration called "Regularized Generalized Canonical Correlation Analysis" (RGCCA [24][25][26][27]) enables identifying such putative biomarkers. This framework is implemented and made freely avalaible within the RGCCA R package .
Multiomics in health and disease
[edit]
Multiomics currently holds a promise to fill gaps in the understanding of human health and disease, and many researchers are working on ways to generate and analyze disease-related data.[28] The applications range from understanding host-pathogen interactions and infectious diseases,[29][30] cancer,[31] to understanding better chronic and complex non-communicable diseases[32] and improving personalized medicine.[33]
Integrated Human Microbiome Project
[edit]The second phase of the $170 million Human Microbiome Project was focused on integrating patient data to different omic datasets, considering host genetics, clinical information and microbiome composition.[34][35] The phase one focused on characterization of communities in different body sites. Phase 2 focused in the integration of multiomic data from host & microbiome to human diseases. Specifically, the project used multiomics to improve the understanding of the interplay of gut and nasal microbiomes with type 2 diabetes,[36] gut microbiomes and inflammatory bowel disease[37] and vaginal microbiomes and pre-term birth.[38]
Systems Immunology
[edit]The complexity of interactions in the human immune system has prompted the generation of a wealth of immunology-related multi-scale omic data.[39] Multi-omic data analysis has been employed to gather novel insights about the immune response to infectious diseases, such as pediatric chikungunya,[40] as well as noncommunicable autoimmune diseases.[41] Integrative omics has also been employed strongly to understand effectiveness and side effects of vaccines, a field called systems vaccinology.[42] For example, multiomics was essential to uncover the association of changes in plasma metabolites and immune system transcriptome on response to vaccination against herpes zoster.[43]
List of software used for multi-omic analysis
[edit]The Bioconductor project curates a variety of R packages aimed at integrating omic data:
- omicade4, for multiple co-inertia analysis of multi omic datasets[44]
- MultiAssayExperiment, offering a bioconductor interface for overlapping samples[45]
- IMAS, a package focused on using multi omic data for evaluating alternative splicing[46]
- bioCancer, a package for visualization of multiomic cancer data[47]
- mixOmics, a suite of multivariate methods for data integration[23]
- MultiDataSet, a package for encapsulating multiple data sets[48]
The RGCCA package implements a versatile framework for data integration. This package is freely available on the Comprehensive R Archive Network (CRAN).
The OmicTools[49] database further highlights R packages and othertools for multi omic data analysis:
- PaintOmics, a web resource for visualization of multi-omics datasets[50][51]
- SIGMA, a Java program focused on integrated analysis of cancer datasets[52]
- iOmicsPASS, a tool in C++ for multiomic-based phenotype prediction[53]
- Grimon, an R graphical interface for visualization of multiomic data[54]
- Omics Pipe, a framework in Python for reproducibly automating multiomic data analysis[55]
Multiomic Databases
[edit]A major limitation of classical omic studies is the isolation of only one level of biological complexity. For example, transcriptomic studies may provide information at the transcript level, but many different entities contribute to the biological state of the sample (genomic variants, post-translational modifications, metabolic products, interacting organisms, among others). With the advent of high-throughput biology, it is becoming increasingly affordable to make multiple measurements, allowing transdomain (e.g. RNA and protein levels) correlations and inferences. These correlations aid the construction or more complete biological networks, filling gaps in our knowledge.
Integration of data, however, is not an easy task. To facilitate the process, groups have curated database and pipelines to systematically explore multiomic data:
- Multi-Omics Profiling Expression Database (MOPED),[56] integrating diverse animal models,
- The Pancreatic Expression Database, integrating data related to pancreatic tissue,
- LinkedOmics,[57][58] connecting data from TCGA cancer datasets,
- OASIS,[59] a web-based resource for general cancer studies,
- BCIP,[60] a platform for breast cancer studies,
- C/VDdb,[61] connecting data from several cardiovascular disease studies,
- ZikaVR,[62] a multiomic resource for Zika virus data
- Ecomics,[63] a normalized multi-omic database for Escherichia coli data,
- GourdBase,[64] integrating data from studies with gourd,
- MODEM,[65] a database for multilevel maize data,
- SoyKB,[66] a database for multilevel soybean data,
- ProteomicsDB,[67] a multi-omics and multi-organism resource for life science research
See also
[edit]References
[edit]- ^ Bersanelli, Matteo; Mosca, Ettore; Remondini, Daniel; Giampieri, Enrico; Sala, Claudia; Castellani, Gastone; Milanesi, Luciano (1 January 2016). "Methods for the integration of multi-omics data: mathematical aspects". BMC Bioinformatics. 17 (2): S15. doi:10.1186/s12859-015-0857-9. ISSN 1471-2105. PMC 4959355. PMID 26821531.
- ^ Bock, Christoph; Farlik, Matthias; Sheffield, Nathan C. (August 2016). "Multi-Omics of Single Cells: Strategies and Applications". Trends in Biotechnology. 34 (8): 605–608. doi:10.1016/j.tibtech.2016.04.004. PMC 4959511. PMID 27212022.
- ^ Vilanova, Cristina; Porcar, Manuel (26 July 2016). "Are multi-omics enough?". Nature Microbiology. 1 (8): 16101. doi:10.1038/nmicrobiol.2016.101. PMID 27573112. S2CID 3835720.
- ^ Tarazona, S., Balzano-Nogueira, L., & Conesa, A. (2018). Multiomics Data Integration in Time Series Experiments. doi:10.1016/bs.coac.2018.06.005
- ^ PSB'14 Cancer Panomics Session Archived 2013-09-23 at the Wayback Machine
- ^ The Molecular Landscape of Cancer: Using Panomics to Drive Change Archived 2013-11-09 at the Wayback Machine
- ^ "Glossary". Accelerating Progress Against Cancer: ASCO's blueprint for transforming clinical and translational cancer research (PDF). American Society of Clinical Oncology. 2011. p. 28. Retrieved 13 September 2013.
- ^ a b MacAulay, Iain C.; Ponting, Chris P.; Voet, Thierry (2017). "Single-Cell Multiomics: Multiple Measurements from Single Cells". Trends in Genetics. 33 (2): 155–168. doi:10.1016/j.tig.2016.12.003. PMC 5303816. PMID 28089370.
- ^ Hu, Youjin; An, Qin; Sheu, Katherine; Trejo, Brandon; Fan, Shuxin; Guo, Ying (2018-04-20). "Single Cell Multi-Omics Technology: Methodology and Application". Frontiers in Cell and Developmental Biology. 6: 28. doi:10.3389/fcell.2018.00028. ISSN 2296-634X. PMC 5919954. PMID 29732369.
- ^ Kester, Lennart Spanjaard, Bastiaan Bienko, Magda van Oudenaarden, Alexander Dey, Siddharth S (2015). "Integrated genome and transcriptome sequencing of the same cell". Nature Biotechnology. 33 (3): 285–289. doi:10.1038/nbt.3129. OCLC 931063996. PMC 4374170. PMID 25599178.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Macaulay, Iain C; Teng, Mabel J; Haerty, Wilfried; Kumar, Parveen; Ponting, Chris P; Voet, Thierry (2016-09-29). "Separation and parallel sequencing of the genomes and transcriptomes of single cells using G&T-seq". Nature Protocols. 11 (11): 2081–2103. doi:10.1038/nprot.2016.138. hdl:20.500.11820/015ce29d-7e2d-42c8-82fa-cb1290b761c0. ISSN 1754-2189. PMID 27685099. S2CID 24351548.
- ^ Tang, Fuchou; Wen, Lu; Li, Xianlong; Wu, Xinglong; Zhu, Ping; Guo, Hongshan (2013-12-01). "Single-cell methylome landscapes of mouse embryonic stem cells and early embryos analyzed using reduced representation bisulfite sequencing". Genome Research. 23 (12): 2126–2135. doi:10.1101/gr.161679.113. ISSN 1088-9051. PMC 3847781. PMID 24179143.
- ^ Kelsey, Gavin; Reik, Wolf; Stegle, Oliver; Andrews, Simon R.; Julian Peat; Saadeh, Heba; Krueger, Felix; Angermueller, Christof; Lee, Heather J. (August 2014). "Single-cell genome-wide bisulfite sequencing for assessing epigenetic heterogeneity". Nature Methods. 11 (8): 817–820. doi:10.1038/nmeth.3035. ISSN 1548-7105. PMC 4117646. PMID 25042786.
- ^ Angermueller, Christof; Clark, Stephen J; Lee, Heather J; Macaulay, Iain C; Teng, Mabel J; Hu, Tim Xiaoming; Krueger, Felix; Smallwood, Sébastien A; Ponting, Chris P (2016-01-11). "Parallel single-cell sequencing links transcriptional and epigenetic heterogeneity". Nature Methods. 13 (3): 229–232. doi:10.1038/nmeth.3728. ISSN 1548-7091. PMC 4770512. PMID 26752769.
- ^ Greenleaf, William J.; Chang, Howard Y.; Snyder, Michael P.; Michael L. Gonzales; Ruff, Dave; Litzenburger, Ulrike M.; Wu, Beijing; Buenrostro, Jason D. (July 2015). "Single-cell chromatin accessibility reveals principles of regulatory variation". Nature. 523 (7561): 486–490. Bibcode:2015Natur.523..486B. doi:10.1038/nature14590. ISSN 1476-4687. PMC 4685948. PMID 26083756.
- ^ Fraser, Peter; Tanay, Amos; Laue, Ernest D.; Dean, Wendy; Yaffe, Eitan; Schoenfelder, Stefan; Stevens, Tim J.; Lubling, Yaniv; Nagano, Takashi (October 2013). "Single-cell Hi-C reveals cell-to-cell variability in chromosome structure". Nature. 502 (7469): 59–64. Bibcode:2013Natur.502...59N. doi:10.1038/nature12593. ISSN 1476-4687. PMC 3869051. PMID 24067610.
- ^ a b Darmanis, Spyros; Gallant, Caroline Julie; Marinescu, Voichita Dana; Niklasson, Mia; Segerman, Anna; Flamourakis, Georgios; Fredriksson, Simon; Assarsson, Erika; Lundberg, Martin (2016-01-12). "Simultaneous Multiplexed Measurement of RNA and Proteins in Single Cells". Cell Reports. 14 (2): 380–389. doi:10.1016/j.celrep.2015.12.021. ISSN 2211-1247. PMC 4713867. PMID 26748716.
- ^ a b Gherardini, Pier Federico; Nolan, Garry P.; Chen, Shih-Yu; Hsieh, Elena W. Y.; Zunder, Eli R.; Bava, Felice-Alessio; Frei, Andreas P. (March 2016). "Highly multiplexed simultaneous detection of RNAs and proteins in single cells". Nature Methods. 13 (3): 269–275. doi:10.1038/nmeth.3742. ISSN 1548-7105. PMC 4767631. PMID 26808670.
- ^ Assarsson, Erika; Lundberg, Martin; Holmquist, Göran; Björkesten, Johan; Bucht Thorsen, Stine; Ekman, Daniel; Eriksson, Anna; Rennel Dickens, Emma; Ohlsson, Sandra (2014-04-22). "Homogenous 96-Plex PEA Immunoassay Exhibiting High Sensitivity, Specificity, and Excellent Scalability". PLOS ONE. 9 (4): e95192. Bibcode:2014PLoSO...995192A. doi:10.1371/journal.pone.0095192. ISSN 1932-6203. PMC 3995906. PMID 24755770.
- ^ Garmire, Lana X.; Chaudhary, Kumardeep; Huang, Sijia (2017). "More Is Better: Recent Progress in Multi-Omics Data Integration Methods". Frontiers in Genetics. 8: 84. doi:10.3389/fgene.2017.00084. ISSN 1664-8021. PMC 5472696. PMID 28670325.
- ^ Tagkopoulos, Ilias; Kim, Minseung (2018). "Data integration and predictive modeling methods for multi-omics datasets". Molecular Omics. 14 (1): 8–25. doi:10.1039/C7MO00051K. PMID 29725673.
- ^ Lin, Eugene; Lane, Hsien-Yuan (2017-01-20). "Machine learning and systems genomics approaches for multi-omics data". Biomarker Research. 5 (1): 2. doi:10.1186/s40364-017-0082-y. ISSN 2050-7771. PMC 5251341. PMID 28127429.
- ^ a b Rohart, Florian; Gautier, Benoît; Singh, Amrit; Lê Cao, Kim-Anh (2017-02-14). "mixOmics: an R package for 'omics feature selection and multiple data integration". PLOS Computational Biology. 13 (11): e1005752. Bibcode:2017PLSCB..13E5752R. bioRxiv 10.1101/108597. doi:10.1371/journal.pcbi.1005752. PMC 5687754. PMID 29099853.
- ^ Tenenhaus, Arthur; Tenenhaus, Michel (2011-03-17). "Regularized Generalized Canonical Correlation Analysis". Psychometrika. 76 (2): 257–284. doi:10.1007/s11336-011-9206-8. ISSN 0033-3123.
- ^ Tenenhaus, A.; Philippe, C.; Guillemot, V.; Le Cao, K.-A.; Grill, J.; Frouin, V. (2014-02-17). "Variable selection for generalized canonical correlation analysis". Biostatistics. 15 (3): 569–583. doi:10.1093/biostatistics/kxu001. ISSN 1465-4644. PMID 24550197.
- ^ Tenenhaus, Arthur; Philippe, Cathy; Frouin, Vincent (October 2015). "Kernel Generalized Canonical Correlation Analysis". Computational Statistics & Data Analysis. 90: 114–131. doi:10.1016/j.csda.2015.04.004. ISSN 0167-9473.
- ^ Tenenhaus, Michel; Tenenhaus, Arthur; Groenen, Patrick J. F. (2017-05-23). "Regularized Generalized Canonical Correlation Analysis: A Framework for Sequential Multiblock Component Methods". Psychometrika. 82 (3): 737–777. doi:10.1007/s11336-017-9573-x. ISSN 0033-3123.
- ^ Hasin, Yehudit; Seldin, Marcus; Lusis, Aldons (2017-05-05). "Multi-omics approaches to disease". Genome Biology. 18 (1): 83. doi:10.1186/s13059-017-1215-1. ISSN 1474-760X. PMC 5418815. PMID 28476144.
- ^ Khan, Mohd M.; Ernst, Orna; Manes, Nathan P.; Oyler, Benjamin L.; Fraser, Iain D. C.; Goodlett, David R.; Nita-Lazar, Aleksandra (2019-03-11). "Multi-Omics Strategies Uncover Host–Pathogen Interactions". ACS Infectious Diseases. 5 (4): 493–505. doi:10.1021/acsinfecdis.9b00080. ISSN 2373-8227. PMID 30857388. S2CID 75137107.
- ^ Aderem, Alan; Adkins, Joshua N.; Ansong, Charles; Galagan, James; Kaiser, Shari; Korth, Marcus J.; Law, G. Lynn; McDermott, Jason G.; Proll, Sean C. (2011-02-01). "A Systems Biology Approach to Infectious Disease Research: Innovating the Pathogen-Host Research Paradigm". mBio. 2 (1): e00325-10. doi:10.1128/mbio.00325-10. ISSN 2150-7511. PMC 3034460. PMID 21285433.
- ^ Mouchtouris, N; Smit, RD; Piper, K; Prashant, G; Evans, JJ; Karsy, M (4 March 2022). "A review of multiomics platforms in pituitary adenoma pathogenesis". Frontiers in Bioscience (Landmark Edition). 27 (3): 77. doi:10.31083/j.fbl2703077. PMID 35345309. S2CID 247560386.
- ^ Yan, Jingwen; Risacher, Shannon L; Shen, Li; Saykin, Andrew J. (2017-06-30). "Network approaches to systems biology analysis of complex disease: integrative methods for multi-omics data". Briefings in Bioinformatics. 19 (6): 1370–1381. doi:10.1093/bib/bbx066. ISSN 1467-5463. PMC 6454489. PMID 28679163.
- ^ He, Feng Q.; Ollert, Markus; Balling, Rudi; Bode, Sebastian F. N.; Delhalle, Sylvie (2018-02-06). "A roadmap towards personalized immunology". npj Systems Biology and Applications. 4 (1): 9. doi:10.1038/s41540-017-0045-9. ISSN 2056-7189. PMC 5802799. PMID 29423275.
- ^ Proctor, Lita M.; Creasy, Heather H.; Fettweis, Jennifer M.; Lloyd-Price, Jason; Mahurkar, Anup; Zhou, Wenyu; Buck, Gregory A.; Snyder, Michael P.; Strauss, Jerome F. (May 2019). "The Integrative Human Microbiome Project". Nature. 569 (7758): 641–648. Bibcode:2019Natur.569..641I. doi:10.1038/s41586-019-1238-8. ISSN 1476-4687. PMC 6784865. PMID 31142853.
- ^ "After the Integrative Human Microbiome Project, what's next for the microbiome community?". Nature. 569 (7758): 599. 2019-05-29. Bibcode:2019Natur.569Q.599.. doi:10.1038/d41586-019-01674-w. PMID 31142868. S2CID 169035865.
- ^ Snyder, Michael; Weinstock, George M.; Sodergren, Erica; McLaughlin, Tracey; Tse, David; Rost, Hannes; Piening, Brian; Kukurba, Kim; Rose, Sophia Miryam Schüssler-Fiorenza (May 2019). "Longitudinal multi-omics of host–microbe dynamics in prediabetes". Nature. 569 (7758): 663–671. Bibcode:2019Natur.569..663Z. doi:10.1038/s41586-019-1236-x. ISSN 1476-4687. PMC 6666404. PMID 31142858.
- ^ Huttenhower, Curtis; Xavier, Ramnik J.; Vlamakis, Hera; Franzosa, Eric A.; Clish, Clary B.; Winter, Harland S.; Stappenbeck, Thaddeus S.; Petrosino, Joseph F.; McGovern, Dermot P. B. (May 2019). "Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases". Nature. 569 (7758): 655–662. Bibcode:2019Natur.569..655L. doi:10.1038/s41586-019-1237-9. ISSN 1476-4687. PMC 6650278. PMID 31142855.
- ^ Buck, Gregory A.; Strauss, Jerome F.; Jefferson, Kimberly K.; Hendricks-Muñoz, Karen D.; Wijesooriya, N. Romesh; Rubens, Craig E.; Gravett, Michael G.; Sexton, Amber L.; Chaffin, Donald O. (June 2019). "The vaginal microbiome and preterm birth". Nature Medicine. 25 (6): 1012–1021. doi:10.1038/s41591-019-0450-2. ISSN 1546-170X. PMC 6750801. PMID 31142849.
- ^ Kidd, Brian A; Peters, Lauren A; Schadt, Eric E; Dudley, Joel T (2014-01-21). "Unifying immunology with informatics and multiscale biology". Nature Immunology. 15 (2): 118–127. doi:10.1038/ni.2787. ISSN 1529-2908. PMC 4345400. PMID 24448569.
- ^ Harris, Eva; Kasarskis, Andrew; Wolinsky, Steven M.; Suaréz-Fariñas, Mayte; Zhu, Jun; Wang, Li; Balmaseda, Angel; Thomas, Guajira P.; Stewart, Michael G. (2018-08-01). "Comprehensive innate immune profiling of chikungunya virus infection in pediatric cases". Molecular Systems Biology. 14 (8): e7862. doi:10.15252/msb.20177862. ISSN 1744-4292. PMC 6110311. PMID 30150281.
- ^ Firestein, Gary S.; Wang, Wei; Gay, Steffen; Ball, Scott T.; Bartok, Beatrix; Boyle, David L.; Whitaker, John W. (2015-04-22). "Integrative Omics Analysis of Rheumatoid Arthritis Identifies Non-Obvious Therapeutic Targets". PLOS ONE. 10 (4): e0124254. Bibcode:2015PLoSO..1024254W. doi:10.1371/journal.pone.0124254. ISSN 1932-6203. PMC 4406750. PMID 25901943.
- ^ Pulendran, Bali; Li, Shuzhao; Nakaya, Helder I. (2010-10-29). "Systems Vaccinology". Immunity. 33 (4): 516–529. doi:10.1016/j.immuni.2010.10.006. ISSN 1074-7613. PMC 3001343. PMID 21029962.
- ^ Li, Shuzhao; Sullivan, Nicole L.; Rouphael, Nadine; Yu, Tianwei; Banton, Sophia; Maddur, Mohan S.; McCausland, Megan; Chiu, Christopher; Canniff, Jennifer (2017-05-18). "Metabolic Phenotypes of Response to Vaccination in Humans". Cell. 169 (5): 862–877.e17. doi:10.1016/j.cell.2017.04.026. ISSN 0092-8674. PMC 5711477. PMID 28502771.
- ^ Meng, Chen; Kuster, Bernhard; Culhane, Aedín C; Gholami, Amin (2014). "A multivariate approach to the integration of multi-omics datasets". BMC Bioinformatics. 15 (1): 162. doi:10.1186/1471-2105-15-162. ISSN 1471-2105. PMC 4053266. PMID 24884486.
- ^ Ramos, Marcel; Schiffer, Lucas; Re, Angela; Azhar, Rimsha; Basunia, Azfar; Rodriguez, Carmen; Chan, Tiffany; Chapman, Phil; Davis, Sean R.; Gomez-Cabrero, David; Culhane, Aedin C.; Haibe-Kains, Benjamin; Hansen, Kasper D.; Kodali, Hanish; Louis, Marie S.; Mer, Arvind S.; Riester, Markus; Morgan, Martin; Carey, Vince; Waldron, Levi (1 November 2017). "Software for the Integration of Multiomics Experiments in Bioconductor". Cancer Research. 77 (21): e39–e42. doi:10.1158/0008-5472.CAN-17-0344. PMC 5679241. PMID 29092936.
- ^ Seonggyun Han, Younghee Lee (2017), IMAS, Bioconductor, doi:10.18129/b9.bioc.imas, retrieved 2019-06-28
- ^ Karim Mezhoud [Aut, Cre] (2017), bioCancer, Bioconductor, doi:10.18129/b9.bioc.biocancer, retrieved 2019-06-28
- ^ Hernandez-Ferrer, Carles; Ruiz-Arenas, Carlos; Beltran-Gomila, Alba; González, Juan R. (2017-01-17). "MultiDataSet: an R package for encapsulating multiple data sets with application to omic data integration". BMC Bioinformatics. 18 (1): 36. doi:10.1186/s12859-016-1455-1. ISSN 1471-2105. PMC 5240259. PMID 28095799.
- ^ "Reap the rewards of a biological insight engine". omicX. Retrieved 2019-06-26.
- ^ Conesa, Ana; Dopazo, Joaquín; García-López, Federico; García-Alcalde, Fernando (2011-01-01). "Paintomics: a web based tool for the joint visualization of transcriptomics and metabolomics data". Bioinformatics. 27 (1): 137–139. doi:10.1093/bioinformatics/btq594. ISSN 1367-4803. PMC 3008637. PMID 21098431.
- ^ Conesa, Ana; Pappas, Georgios J.; Furió-Tarí, Pedro; Balzano-Nogueira, Leandro; Martínez-Mira, Carlos; Tarazona, Sonia; Hernández-de-Diego, Rafael (2018-07-02). "PaintOmics 3: a web resource for the pathway analysis and visualization of multi-omics data". Nucleic Acids Research. 46 (W1): W503–W509. doi:10.1093/nar/gky466. ISSN 0305-1048. PMC 6030972. PMID 29800320.
- ^ Chari, Raj; Coe, Bradley P.; Wedseltoft, Craig; Benetti, Marie; Wilson, Ian M.; Vucic, Emily A.; MacAulay, Calum; Ng, Raymond T.; Lam, Wan L. (2008-10-07). "SIGMA2: A system for the integrative genomic multi-dimensional analysis of cancer genomes, epigenomes, and transcriptomes". BMC Bioinformatics. 9 (1): 422. doi:10.1186/1471-2105-9-422. ISSN 1471-2105. PMC 2571113. PMID 18840289.
- ^ Choi, Hyungwon; Ewing, Rob; Choi, Kwok Pui; Fermin, Damian; Koh, Hiromi W. L. (2018-07-23). "iOmicsPASS: a novel method for integration of multi-omics data over biological networks and discovery of predictive subnetworks". bioRxiv: 374520. doi:10.1101/374520. S2CID 92157115.
- ^ Kanai, Masahiro; Maeda, Yuichi; Okada, Yukinori (2018-06-19). "Grimon: graphical interface to visualize multi-omics networks". Bioinformatics. 34 (22): 3934–3936. doi:10.1093/bioinformatics/bty488. ISSN 1367-4803. PMC 6223372. PMID 29931190.
- ^ Su, Andrew I.; Loguercio, Salvatore; Carland, Tristan M.; Ducom, Jean-Christophe; Gioia, Louis; Meißner, Tobias; Fisch, Kathleen M. (2015-06-01). "Omics Pipe: a community-based framework for reproducible multi-omics data analysis". Bioinformatics. 31 (11): 1724–1728. doi:10.1093/bioinformatics/btv061. ISSN 1367-4803. PMC 4443682. PMID 25637560.
- ^ Montague, Elizabeth; Stanberry, Larissa; Higdon, Roger; Janko, Imre; Lee, Elaine; Anderson, Nathaniel; Choiniere, John; Stewart, Elizabeth; Yandl, Gregory (June 2014). "MOPED 2.5—An Integrated Multi-Omics Resource: Multi-Omics Profiling Expression Database Now Includes Transcriptomics Data". OMICS: A Journal of Integrative Biology. 18 (6): 335–343. doi:10.1089/omi.2014.0061. ISSN 1536-2310. PMC 4048574. PMID 24910945.
- ^ Zhang, Bing; Wang, Jing; Straub, Peter; Vasaikar, Suhas V. (2018-01-04). "LinkedOmics: analyzing multi-omics data within and across 32 cancer types". Nucleic Acids Research. 46 (D1): D956–D963. doi:10.1093/nar/gkx1090. ISSN 0305-1048. PMC 5753188. PMID 29136207.
- ^ "LinkedOmics :: Login". www.linkedomics.org. Retrieved 2019-06-26.
- ^ Kan, Zhengyan; Rejto, Paul A.; Roberts, Peter; Ding, Ying; AChing, Keith; Wang, Kai; Deng, Shibing; Schefzick, Sabine; Estrella, Heather (January 2016). "OASIS: web-based platform for exploring cancer multi-omics data". Nature Methods. 13 (1): 9–10. doi:10.1038/nmeth.3692. ISSN 1548-7105. PMID 26716558. S2CID 38621277.
- ^ Wu, Jiaqi; Hu, Shuofeng; Chen, Yaowen; Li, Zongcheng; Zhang, Jian; Yuan, Hanyu; Shi, Qiang; Shao, Ningsheng; Ying, Xiaomin (May 2017). "BCIP: a gene-centered platform for identifying potential regulatory genes in breast cancer". Scientific Reports. 7 (1): 45235. Bibcode:2017NatSR...745235W. doi:10.1038/srep45235. ISSN 2045-2322. PMC 5361122. PMID 28327601.
- ^ Husi, Holger; Patel, Alisha; Fernandes, Marco (2018-11-12). "C/VDdb: A multi-omics expression profiling database for a knowledge-driven approach in cardiovascular disease (CVD)". PLOS ONE. 13 (11): e0207371. Bibcode:2018PLoSO..1307371F. doi:10.1371/journal.pone.0207371. ISSN 1932-6203. PMC 6231654. PMID 30419069.
- ^ Gupta, Amit Kumar; Kaur, Karambir; Rajput, Akanksha; Dhanda, Sandeep Kumar; Sehgal, Manika; Khan, Md. Shoaib; Monga, Isha; Dar, Showkat Ahmad; Singh, Sandeep (2016-09-16). "ZikaVR: An Integrated Zika Virus Resource for Genomics, Proteomics, Phylogenetic and Therapeutic Analysis". Scientific Reports. 6 (1): 32713. Bibcode:2016NatSR...632713G. doi:10.1038/srep32713. ISSN 2045-2322. PMC 5025660. PMID 27633273.
- ^ Tagkopoulos, Ilias; Violeta Zorraquino; Rai, Navneet; Kim, Minseung (2016-10-07). "Multi-omics integration accurately predicts cellular state in unexplored conditions for Escherichia coli". Nature Communications. 7: 13090. Bibcode:2016NatCo...713090K. doi:10.1038/ncomms13090. ISSN 2041-1723. PMC 5059772. PMID 27713404.
- ^ Li, Guojing; Lu, Zhongfu; Lin, Jiandong; Hu, Yaowen; Yunping Huang; Wang, Baogen; Wu, Xinyi; Wu, Xiaohua; Xu, Pei (2018-02-26). "GourdBase: a genome-centered multi-omics database for the bottle gourd ( Lagenaria siceraria ), an economically important cucurbit crop". Scientific Reports. 8 (1): 3604. Bibcode:2018NatSR...8.3604W. doi:10.1038/s41598-018-22007-3. ISSN 2045-2322. PMC 5827520. PMID 29483591.
- ^ Liu, Haijun; Wang, Fan; Xiao, Yingjie; Tian, Zonglin; Wen, Weiwei; Zhang, Xuehai; Chen, Xi; Liu, Nannan; Li, Wenqiang (2016). "MODEM: multi-omics data envelopment and mining in maize". Database. 2016: baw117. doi:10.1093/database/baw117. ISSN 1758-0463. PMC 4976297. PMID 27504011.
- ^ Xu, Dong; Nguyen, Henry T.; Stacey, Gary; Gaudiello, Eric C.; Endacott, Ryan Z.; Zhang, Hongxin; Liu, Yang; Chen, Shiyuan; Fitzpatrick, Michael R. (2014-01-01). "Soybean knowledge base (SoyKB): a web resource for integration of soybean translational genomics and molecular breeding". Nucleic Acids Research. 42 (D1): D1245–D1252. doi:10.1093/nar/gkt905. ISSN 0305-1048. PMC 3965117. PMID 24136998.
- ^ Samaras, Patroklos; Schmidt, Tobias; Frejno, Martin; Gessulat, Siegfried; Reinecke, Maria; Jarzab, Anna; Zecha, Jana; Mergner, Julia; Giansanti, Piero; Ehrlich, Hans-Christian; Aiche, Stephan (2020-01-08). "ProteomicsDB: a multi-omics and multi-organism resource for life science research". Nucleic Acids Research. 48 (D1): D1153–D1163. doi:10.1093/nar/gkz974. ISSN 0305-1048. PMC 7145565. PMID 31665479.
