Perfluorohexyloctane
 | |
| Clinical data | |
|---|---|
| Trade names | Evotears Miebo (/ˈmaɪboʊ/ MY-bow) Novatears |
| Other names | NOV03; 1-(perfluorohexyl)octane |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a623054 |
| License data | |
| Routes of administration | Eye drops |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
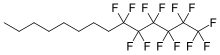
| Formula | C14H17F13 |
| Molar mass | 432.269 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Perfluorohexyloctane (branded as Evotears, Miebo,[a] and Novatears, among others) is a medication used for the treatment of dry eye disease.[4] It is a semifluorinated alkane.[4]
Perfluorohexyloctane has been available in multiple markets since 2015 under the brand names Evotears and Novatears,[5] and was additionally approved for medical use in the United States in May 2023 under the brand name Miebo.[4][6] The US Food and Drug Administration (FDA) considers it to be a first-in-class medication.[7]
Medical uses
[edit]Perfluorohexyloctane is indicated for the treatment of the signs and symptoms of dry eye disease.[4][8][9]
Availability
[edit]Perfluorohexyloctane is sold as an over-the-counter medication under the brand names Evotears and Novatears in multiple countries,[10] costing around NZ$34.00, A$30, and €30 for a one-month supply.
In the US, perfluorohexyloctane is sold under the brand name Miebo; a prescription is required.
See also
[edit]Notes and references
[edit]- ^ "Notice: Multiple additions to the Prescription Drug List (PDL) [2024-10-18]". Health Canada. 18 October 2024. Retrieved 25 October 2024.
- ^ "Miebo product information". Health Canada. 4 September 2024. Retrieved 27 December 2024.
- ^ "Regulatory Decision Summary for Miebo". Drug and Health Products Portal. 4 September 2024. Retrieved 27 December 2024.
- ^ a b c d e "Miebo- perfluorohexyloctane solution". DailyMed. 18 May 2023. Retrieved 8 June 2023.
- ^ "URSAPHARM GmbH and Novaliq GmbH Announce European Partnership Agreement" (Press release). Retrieved 15 February 2024.
- ^ "Bausch + Lomb and Novaliq Announce FDA Approval of Miebo (Perfluorohexyloctane Ophthalmic Solution) for the Treatment of the Signs and Symptoms of Dry Eye Disease" (Press release). Bausch + Lomb Corporation. 18 May 2023. Retrieved 8 June 2023 – via Business Wire.
- ^ New Drug Therapy Approvals 2023 (PDF). U.S. Food and Drug Administration (FDA) (Report). January 2024. Archived from the original on 10 January 2024. Retrieved 9 January 2024.
- ^ Ballesteros-Sánchez A, De-Hita-Cantalejo C, Sánchez-González MC, Jansone-Langine Z, de Sotomayor MA, Culig J, et al. (October 2023). "Perfluorohexyloctane in dry eye disease: A systematic review of its efficacy and safety as a novel therapeutic agent". The Ocular Surface. 30: 254–262. doi:10.1016/j.jtos.2023.10.001. hdl:11441/151762. PMID 37813152. S2CID 263802332.
- ^ Sheppard JD, Evans DG, Protzko EE (November 2023). "A review of the first anti-evaporative prescription treatment for dry eye disease: perfluorohexyloctane ophthalmic solution". The American Journal of Managed Care. 29 (14 Suppl): S251 – S259. doi:10.37765/ajmc.2023.89464. PMID 37930231. S2CID 265032840.
- ^ "In Australia, NovaTears Eye Drops Are Available on the Pharmaceutical Benefits Scheme (PBS) from Now On" (Press release). Retrieved 15 February 2024.
Further reading
[edit]- Azhar A, Taimuri MA, Oduoye MO, Sumbal A, Sheikh A, Iqbal A, et al. (September 2024). "MEIBO (perfluorohexyloctane): a novel approach to treating dry eye disease". Annals of Medicine and Surgery (2012). 86 (9): 5292–5298. doi:10.1097/MS9.0000000000002322. PMC 11374244. PMID 39239035.
