Mammalian reproduction

Most mammals are viviparous, giving birth to live young.[1] However, the five species of monotreme, the platypuses and the echidnas, lay eggs. The monotremes have a sex determination system different from that of most other mammals.[2] In particular, the sex chromosomes of a platypus are more like those of a chicken than those of a therian mammal.[3]
The mammary glands of mammals are specialized to produce milk, a liquid used by newborns as their primary source of nutrition. The monotremes branched early from other mammals and do not have the teats seen in most mammals, but they do have mammary glands. The young lick the milk from a mammary patch on the mother's belly.
Viviparous mammals are in the subclass Theria; those living today are in the Marsupialia and Placentalia infraclasses. A marsupial has a short gestation period, typically shorter than its estrous cycle, and gives birth to an underdeveloped (altricial) newborn that then undergoes further development; in many species, this takes place within a pouch-like sac, the marsupium, located in the front of the mother's abdomen. Some placentals, e.g. guinea pig, give birth to fully developed (precocial) young, usually after long gestation periods, while some others, e.g. mouse, give birth to underdeveloped young.
Maturity and reproductive age
[edit]Sexual maturity and thus the earliest age at which mammals can reproduce varies dramatically across species. Members of the rodent family Cricetidae can reach sexual maturity in 1–2 months, e.g. the Norway lemming (Lemmus lemmus) in 39 days. Many dogs (family Canidae) and bovids (Bovidae) take about a year to reach maturity while primates (including humans) and dolphins (Delphinidae) require more than 10 years. Some whales take even longer, with the longest duration being recorded for the bowhead whale (Balaena mysticetus), which reaches maturity at an age of only about 23 years.[4]
Reproductive system
[edit]Placental mammals
[edit]
Male placentals
[edit]The mammalian male reproductive system contains two main divisions, the penis and the testicles, the latter of which are where sperm are produced and usually held in a scrotum.[5] In humans, both of these organs are outside the abdominal cavity, but they can be primarily housed within the abdomen in other animals. For instance, a dog's penis is covered by a penile sheath except when mating. Having the testicles outside the abdomen best facilitates temperature regulation of the sperm, which require specific temperatures to survive. The external location may also cause a reduction in the heat-induced contribution to the spontaneous mutation rate in male germinal tissue.[6] Sperm are the smaller of the two gametes and are generally very short-lived, requiring males to produce them continuously from the time of sexual maturity until death. The produced sperm are stored in the epididymides until ejaculation through the vasa deferentia. The sperm cells are motile and they swim using tail-like flagella to propel themselves towards the ovum. The sperm follows temperature gradients (thermotaxis)[7] and chemical gradients (chemotaxis) to locate the ovum.
Female placentals
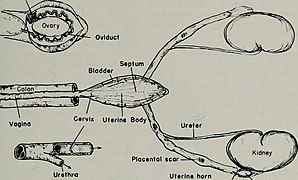
[edit]The mammalian female reproductive system contains three main divisions: the vagina and uterus, which act as the receptacle for the sperm, the ovaries, which produce the female's ova, and the vulva, which consists of the labia and clitoris. The vagina, uterus and ovaries are always internal while the vulva is external. The vagina is attached to the uterus through the cervix, while the uterus is attached to the ovaries via the oviducts. At certain intervals, the ovaries release an ovum, which passes through the oviduct into the uterus.
If, in this transit, it meets with sperm, the egg selects sperm with which to merge; this is termed fertilization. The fertilization usually occurs in the oviducts, but can happen in the uterus itself. The zygote then implants itself in the wall of the uterus, where it begins the processes of embryogenesis and morphogenesis. When developed enough to survive outside the womb, the cervix dilates and contractions of the uterus propel the fetus through the birth canal, which is the vagina.
The ova, which are the female sex cells, are much larger than the sperm and are normally formed within the ovaries of the fetus before its birth. They are mostly fixed in location within the ovary until their transit to the uterus, and contain nutrients for the later zygote and embryo. Over a regular interval, in response to hormonal signals, a process of oogenesis matures one ovum which is released and sent down the oviduct. If not fertilized, this egg is released through menstruation in humans and other great apes, and reabsorbed in other mammals in the estrus cycle.
Gestation
[edit]
Gestation, called pregnancy in humans, is the period of time during which the fetus develops, dividing via mitosis inside the female. During this time, the fetus receives all of its nutrition and oxygenated blood from the female, filtered through the placenta, which is attached to the fetus' abdomen via an umbilical cord. This drain of nutrients can be quite taxing on the female, who is required to ingest slightly higher levels of calories. In addition, certain vitamins and other nutrients are required in greater quantities than normal, often creating abnormal eating habits. The length of gestation, called the gestation period, varies greatly from species to species; it is 40 weeks in humans, 56–60 in giraffes and 16 days in hamsters.
Birth
[edit]Once the fetus has sufficiently developed, chemical signals start the process of birth. This begins with contractions of the uterus and dilation of the cervix. The fetus then descends to the cervix, where it is pushed out into the vagina, and eventually out of the female. The newborn, which is called an infant in humans, should typically begin respiration on its own shortly after birth. Not long after, the placenta is passed as well.
Human births
[edit]Human babies are unique in the animal kingdom due to their large head size relative to their bodies. This has an effect on the birthing process for humans as the bipedal gait of a human causes the birthing canal to be relatively narrow and twisted in the middle. As a result, the vast majority of human babies must rotate inside the birth canal in order to squeeze through the birthing canal and fit through the pelvic planes. This process is known as a rotational birth, and while it is not a process unique to humans, humans are unique in that nearly all human babies undergo this process out of necessity. A primary hypothesis for why this process and others occur, causing human births to be drastically more difficult than other mammals is known as the obstetrical dilemma.[8]
Monotremes
[edit]Monotremes, only five species of which exist, all from Australia and New Guinea, are mammals that lay eggs. They have one opening for excretion and reproduction called the cloaca. They hold the eggs internally for several weeks, providing nutrients, and then lay them and cover them like birds. Like marsupial "joeys", monotreme "puggles" are larval and fetus-like,[9] as like them they cannot expand their torso due to the presence of epipubic bones, forcing them to produce undeveloped young.
Marsupials
[edit]Marsupials' reproductive systems differ markedly from those of placentals,[10][11] though it is probably the plesiomorphic condition found in viviparous mammals, including non-placental eutherians.[12] During embryonic development, a choriovitelline placenta forms in all marsupials. In bandicoots, an additional chorioallantoic placenta forms, although it lacks the chorionic villi found in eutherian placentas.
-
Male domestic cat
-
Female domestic cat
-
Female raccoon
-
Male raccoon
-
Male and female spotted hyena
-
Female marsupials
-
Male rabbit
-
Female rabbit
Gametogenesis
[edit]Animals, including mammals, produce gametes (sperm and egg) through meiosis in gonads (testicles in males and ovaries in females). Sperm are produced by the process of spermatogenesis and eggs are produced by oogenesis. These processes are outlined in the article gametogenesis. During gametogenesis in mammals many genes encoding proteins that take part in DNA repair mechanisms show enhanced or specialized expression [13] These mechanisms include meiotic homologous recombinational repair and mismatch repair.
Copulation
[edit]Sexual behavior can be classified into behavioral states associated with reward motivation ("wanting"), reward consummation also known as pleasure ("liking"), and satiety ("inhibition");[14] these behavioral states are regulated in mammals by reward-based sexual learning, fluctuations in various neurochemicals (i.e., dopamine − sexual desire also known as "wanting"; norepinephrine − sexual arousal; oxytocin and melanocortins − sexual attraction), and gonadal hormone cycles and further influenced by sex pheromones and motor reflexes (i.e., lordosis behaviour) in some mammals.[14][15]
These behavioral states correlate with the phases of the human sexual response cycle: motivation − excitement; consummation − plateau and orgasm; satiety − refraction.[14] Sexual learning (a form of associative learning) occurs when an animal starts to associate bodily features, personality, contextual cues, and other stimuli with genitally-induced sexual pleasure.[14][15] Once formed, these associations in turn impinge upon both sexual wanting and sexual liking.
In most female mammals, the act of copulation is controlled by several innate neurobiological processes, including the motor sexual reflex of lordosis.[16] In males, the act of copulation is more complex, because some learning is necessary, but the innate processes (retrocontrol of penis intromission in the vagina, rhythmic movement of the pelvis, detection of female pheromones) are specific to copulation. These innate processes direct heterosexual copulation.[17] Female lordosis behaviour became secondary in Hominidae and is non-functional in humans.[18] Mammals usually copulate in a dorso-ventral posture, although some primate species copulate in a ventro-vental posture.[19][20]
Most mammals possess a vomeronasal organ that is involved in pheromone detection, including sex pheromones.[21] Despite the fact that humans do not possess this organ, adult humans appear to be sensitive to certain mammalian pheromones that putative pheromone receptor proteins in the olfactory epithelium are capable of detecting.[note 1][21] While sex pheromones clearly play a role in modifying sexual behavior in some mammals, the capacity for general pheromone detection and the involvement of pheromones in human sexual behavior has not yet been determined.[14]
The duration of copulation varies significantly between mammal species,[25] and may be correlated with body mass, lasting longer in large mammals than in small mammals.[26] The duration of copulation may also be correlated with the length of the baculum in mammals.[27]
Male mammals ejaculate semen through the penis into the female reproductive tract during copulation.[28][29] Ejaculation usually occurs after only one intromission in humans, canids, and ungulates, but occurs after multiple intromissions in most mammal species.[30][31]
Copulation can induce ovulation in mammal species that do not ovulate spontaneously.[32]
-
Copulating brown bears
-
Copulating lions
-
Copulating grey kangaroos
-
Copulating Aldabra giant tortoises
-
Copulating Magellanic penguins
See also
[edit]- Mammal reproductive system
- Evolution of descended testes in mammals
- Sexual reproduction § Mammals
- Sex organ § Mammals
- Human reproduction
- Animal sexual behavior § Mammals
- Pregnancy (mammals)
- Equine reproductive system
- Carnivora § Reproductive system
- Canine reproduction
- Dolphin § Reproduction and sexuality
- Llama § Reproduction
- Domestic sheep reproduction
Notes
[edit]- ^ In humans and other animals, trace amine-associated receptors (TAARs) that are expressed in the olfactory epithelium function as olfactory receptors that detect volatile amine odorants, including certain pheromones;[22][23] these TAARs putatively function as a class of pheromone receptors involved in the olfactive detection of social cues.[22][23]
A review of studies involving non-human animals indicated that TAARs in the olfactory epithelium can mediate attractive or aversive behavioral responses to an agonist.[22] This review also noted that the behavioral response evoked by a TAAR can vary across species.[22] For example, TAAR5 mediates attraction to trimethylamine in mice and aversion to trimethylamine in rats.[22] In humans, hTAAR5 presumably mediates aversion to trimethylamine, which is known to act as an hTAAR5 agonist and to possess a foul, fishy odor that is aversive to humans;[22][24] however, hTAAR5 is not the only olfactory receptor that is responsible for trimethylamine olfaction in humans.[22][24] As of December 2015,[update] hTAAR5-mediated trimethylamine aversion has not been examined in published research.[24]
References
[edit]- ^ Preston, Elizabeth (13 February 2024). "Self-Love Is Important, but We Mammals Are Stuck With Sex - Some female birds, reptiles and other animals can make a baby on their own. But for mammals like us, eggs and sperm need each other". The New York Times. Archived from the original on 13 February 2024. Retrieved 16 February 2024.
- ^ Wallis MC, Waters PD, Delbridge ML, Kirby PJ, Pask AJ, Grützner F, Rens W, Ferguson-Smith MA, Graves JA, et al. (2007). "Sex determination in platypus and echidna: autosomal location of SOX3 confirms the absence of SRY from monotremes". Chromosome Research. 15 (8): 949–959. doi:10.1007/s10577-007-1185-3. PMID 18185981. S2CID 812974.
- ^ Marshall Graves, Jennifer A. (2008). "Weird Animal Genomes and the Evolution of Vertebrate Sex and Sex Chromosomes" (PDF). Annual Review of Genetics. 42: 568–586. doi:10.1146/annurev.genet.42.110807.091714. PMID 18983263. Archived from the original (PDF) on 2012-09-04.
- ^ Pacifici, Michela; Santini, Luca; Marco, Moreno Di; Baisero, Daniele; Francucci, Lucilla; Marasini, Gabriele Grottolo; Visconti, Piero; Rondinini, Carlo (2013-11-13). "Generation length for mammals". Nature Conservation. 5: 89–94. doi:10.3897/natureconservation.5.5734. ISSN 1314-3301. Archived from the original on 2021-01-26. Retrieved 2021-03-03.
- ^ Hyman, Libbie Henrietta (1992-09-15). Hyman's Comparative Vertebrate Anatomy. University of Chicago Press. ISBN 978-0-226-87013-7.
- ^ Baltz, RH; Bingham, PM; Drake, JW (1976). "Heat mutagenesis in bacteriophage T4: The transition pathway". Proceedings of the National Academy of Sciences of the United States of America. 73 (4): 1269–73. Bibcode:1976PNAS...73.1269B. doi:10.1073/pnas.73.4.1269. PMC 430244. PMID 4797.
- ^ Bahat, Anat; Tur-Kaspa, Ilan; Gakamsky, Anna; Giojalas, Laura C.; Breitbart, Haim; Eisenbach, Michael (2003). "Thermotaxis of mammalian sperm cells: A potential navigation mechanism in the female genital tract". Nature Medicine. 9 (2): 149–50. doi:10.1038/nm0203-149. hdl:11336/66658. PMID 12563318. S2CID 36538049.
- "Sperm Use Heat Sensors To Find The Egg; Weizmann Institute Research Contributes To Understanding Of Human Fertilization". ScienceDaily (Press release). 3 February 2003.
- ^ Trevathan, Wenda (2015-03-05). "Primate pelvic anatomy and implications for birth". Philosophical Transactions of the Royal Society B: Biological Sciences. 370 (1663): 20140065. doi:10.1098/rstb.2014.0065. ISSN 0962-8436. PMC 4305166. PMID 25602069.
- ^ Manger, Paul R.; Hall, Leslie S.; Pettigrew, John D. (1998). "The development of the external features of the platypus (Ornithorhynchus anatinus)". Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 353 (1372): 1115–1125. doi:10.1098/rstb.1998.0270. PMC 1692310. PMID 9720109.
- ^ Australian Mammal Society (December 1978). Australian Mammal Society. Australian Mammal Society.
- ^ Iowa State University Biology Dept. Discoveries about Marsupial Reproduction Anna King 2001. webpage Archived 2012-09-05 at the Wayback Machine (note shows code, html extension omitted)
- ^ Giallombardo, Andres, 2009 New Cretaceous mammals from Mongolia and the early diversification of Eutheria Ph.D. dissertation, Columbia University, 2009402 pages; AAT 3373736 (abstract) The origin of Placental Mammals, Cimolestidae, Zalambdalestidae
- ^ Baarends WM, van der Laan R, Grootegoed JA (2001). "DNA repair mechanisms and gametogenesis". Reproduction. 121 (1): 31–9. doi:10.1530/reprod/121.1.31. hdl:1765/9599. PMID 11226027.
- ^ a b c d e Georgiadis JR, Kringelbach ML, Pfaus JG (September 2012). "Sex for fun: a synthesis of human and animal neurobiology". Nature Reviews. Urology. 9 (9): 486–98. doi:10.1038/nrurol.2012.151. PMID 22926422. S2CID 13813765.
The sexual pleasure cycle adheres to the basic structure of pleasure cycles related to other rewards (such as food), and can therefore also be expressed in terms of motivation–consummation–satiety or wanting–liking–inhibition (Figure 1; Box 2).6,11,1 ... Similar to other forms of learning, sexual behaviour develops over time as people learn to associate stimuli such as bodily features, personality, and contextual cues with genitally-induced sexual pleasure.7 Adolescence is arguably the most critical phase in sexual development ... Popular belief holds that humans also respond to some distal sexual incentive stimuli (breasts, pheromones) in an unconditioned manner, but this has been difficult to evaluate empirically (Box 1) ... Sexual wanting in both rats and humans involves interaction between gonadal hormones and external stimuli that become sexual incentives through association with genitally-induced sexual reward; pleasurable genital stimulation is thus a major factor in sexual learning ...
• Unconditioned sexual stimuli (that is, those for which the pleasurable effect requires no learning) include proximal genital tactile stimulation in humans and distal stimuli such as pheromones, odours, and certain auditory vocalizations in rats.7,16
• Sexual inhibition involves similar brain mechanisms in rats and humans
• Rats show a similar pattern of brain activation to humans in response to cues related to sexual reward
• Cortical, limbic, hypothalamic, and cerebellar regions are activated by sex-related stimuli in both humans and rats - ^ a b Schultz W (2015). "Neuronal reward and decision signals: from theories to data". Physiological Reviews. 95 (3): 853–951. doi:10.1152/physrev.00023.2014. PMC 4491543. PMID 26109341.
Sexual behavior follows hormonal imbalances, at least in men, but is also strongly based on pleasure. To acquire and follow these primary alimentary and mating rewards is the main reason why the brain's reward system has evolved in the first place. Note that "primary" reward does not refer to the distinction between unconditioned versus conditioned reward; indeed, most primary rewards are learned and thus conditioned (foods are primary rewards that are typically learnt). ... Pleasure is not only one of the three main reward functions but also provides a definition of reward. As homeostasis explains the functions of only a limited number of rewards, the prevailing reason why particular stimuli, objects, events, situations, and activities are rewarding may be pleasure. This applies first of all to sex (who would engage in the ridiculous gymnastics of reproductive activity if it were not for the pleasure) and to the primary homeostatic rewards of food and liquid, and extends to money, taste, beauty, social encounters and nonmaterial, internally set, and intrinsic rewards. ... Desire makes behavior purposeful and directs it towards identifiable goals. Thus desire is the emotion that helps to actively direct behavior towards known rewards, whereas pleasure is the passive experience that derives from a received or anticipated reward. Desire has multiple relations to pleasure; it may be pleasant in itself (I feel a pleasant desire), and it may lead to pleasure (I desire to obtain a pleasant object). Thus pleasure and desire have distinctive characteristics but are closely intertwined. They constitute the most important positive emotions induced by rewards. They prioritize our conscious processing and thus constitute important components of behavioral control. These emotions are also called liking (for pleasure) and wanting (for desire) in addiction research (471) and strongly support the learning and approach generating functions of reward. ... Some of the stimuli and events that are pleasurable in humans may not even evoke pleasure in animals but act instead through innate mechanisms. We simply do not know. Nevertheless, the invention of pleasure and desire by evolution had the huge advantage of allowing a large number of stimuli, objects, events, situations, and activities to be attractive. This mechanism importantly supports the primary reward functions in obtaining essential substances and mating partners.
- ^ Pfaff Donald W., Schwartz-Giblin Susan, Maccarthy Margareth M., Kow Lee-Ming : Cellular and molecular mechanisms of female reproductive behaviors, in Knobil Ernest, Neill Jimmy D. : The physiology of reproduction, Raven Press, 2nd edition, 1994
- ^ Meisel Robert L., Sachs Benjamin D. : The physiology of male sexual behavior. In Knobil Ernest, Neill Jimmy D. The physiology of reproduction, Raven Press, 2nd edition, 1994
- ^ Dixson A.F. Primate sexuality: Comparative studies of the Prosimians, Monkeys, Apes, and Human Beings. Oxford University Press, 2nd edition, 2012.
- ^ Smith, Robert L. (2012-12-02). Sperm Competition and the Evolution of Animal Mating systems. Elsevier. ISBN 978-0-323-14313-4.
- ^ Dixson, Alan F. (2009-05-15). Sexual Selection and the Origins of Human Mating Systems. OUP Oxford. ISBN 978-0-19-156973-9.
- ^ a b Nei M, Niimura Y, Nozawa M (December 2008). "The evolution of animal chemosensory receptor gene repertoires: roles of chance and necessity". Nature Reviews. Genetics. 9 (12): 951–63. doi:10.1038/nrg2480. PMID 19002141. S2CID 11135361.
OR genes are predominantly expressed in sensory neurons of the main olfactory epithelium (MOE) in the nasal cavity. Mammals detect many types of chemicals in the air and some in the water as odorants, whereas fishes recognize water-soluble molecules, such as amino acids, bile acids, sex steroids and prostaglandins. Some mammalian OR genes are known to be expressed in other tissues, including the testis, tongue, brain and placenta17. However, the functional significance of such 'ectopic expression' of OR genes is not definitively known. TAARs are also expressed in the MOE. These receptors were first identified as brain receptors for the trace amines, a collection of amines that are present at low concentrations in the central nervous system18. TAARs were originally suspected to be involved in psychiatric disorders19 but are now known to function as a second class of olfactory receptors10. Some mouse TAARs recognize volatile amines that are present in urine, and it seems that the TAARs function to detect ligands associated with social cues10. ... Most mammals possess an additional olfactory organ called the vomeronasal organ (VnO). ... The VnO was previously thought to be a specialized organ for pheromone detection, but it is now known that the VnO and MOE share some overlapping functions22. ... However, at least one of the five V1R genes is expressed in the human olfactory mucosa72. Furthermore, a recent study suggests that these five genes can activate an OR-like signal transduction pathway in a heterologous expression system73. It is therefore possible that the products of these genes function as pheromone or olfactory receptors. Adult humans do not have a VnO but seem to be sensitive to pheromones74. Another interesting observation is that chickens have no functional or non-functional V1R and V2R genes or a VnO75, although birds use pheromones for mate choice and other behaviours76. It is possible that some OR genes in the MOE are able to detect pheromones, as in humans74,77.
- ^ a b c d e f g Liberles SD (October 2015). "Trace amine-associated receptors: ligands, neural circuits, and behaviors". Curr. Opin. Neurobiol. 34: 1–7. doi:10.1016/j.conb.2015.01.001. PMC 4508243. PMID 25616211.
Roles for another receptor are supported by TAAR5-independent trimethylamine anosmias in humans [32]. ... Several TAARs detect volatile and aversive amines, but the olfactory system is capable of discarding ligand-based or function-based constraints on TAAR evolution. Particular TAARs have mutated to recognize new ligands, with almost an entire teleost clade losing the canonical amine-recognition motif. Furthermore, while some TAARs detect aversive odors, TAAR-mediated behaviors can vary across species. ... The ability of particular TAARs to mediate aversion and attraction behavior provides an exciting opportunity for mechanistic unraveling of odor valence encoding.
Figure 2: Table of ligands, expression patterns, and species-specific behavioral responses for each TAAR - ^ a b "Trace amine receptor: Introduction". International Union of Basic and Clinical Pharmacology. Archived from the original on 23 February 2014. Retrieved 15 February 2014.
Importantly, three ligands identified activating mouse Taars are natural components of mouse urine, a major source of social cues in rodents. Mouse Taar4 recognizes β-phenylethylamine, a compound whose elevation in urine is correlated with increases in stress and stress responses in both rodents and humans. Both mouse Taar3 and Taar5 detect compounds (isoamylamine and trimethylamine, respectively) that are enriched in male versus female mouse urine. Isoamylamine in male urine is reported to act as a pheromone, accelerating puberty onset in female mice [34]. The authors suggest the Taar family has a chemosensory function that is distinct from odorant receptors with a role associated with the detection of social cues. ... The evolutionary pattern of the TAAR gene family is characterized by lineage-specific phylogenetic clustering [26,30,35]. These characteristics are very similar to those observed in the olfactory GPCRs and vomeronasal (V1R, V2R) GPCR gene families.
- ^ a b c Wallrabenstein I, Singer M, Panten J, Hatt H, Gisselmann G (2015). "Timberol® Inhibits TAAR5-Mediated Responses to Trimethylamine and Influences the Olfactory Threshold in Humans". PLOS ONE. 10 (12): e0144704. Bibcode:2015PLoSO..1044704W. doi:10.1371/journal.pone.0144704. PMC 4684214. PMID 26684881.
While mice produce gender-specific amounts of urinary TMA levels and were attracted by TMA, this odor is repellent to rats and aversive to humans [19], indicating that there must be species-specific functions. ... Furthermore, a homozygous knockout of murine TAAR5 abolished the attraction behavior to TMA [19]. Thus, it is concluded that TAAR5 itself is sufficient to mediate a behavioral response at least in mice. ... Whether the TAAR5 activation by TMA elicits specific behavioral output like avoidance behavior in humans still needs to be examined.
- ^ Naguib, Marc (2020-04-19). Advances in the Study of Behavior. Academic Press. ISBN 978-0-12-820726-0.
- ^ Stallmann, Robert R., and A. H. Harcourt. "Size matters: the (negative) allometry of copulatory duration in mammals Archived 2022-04-20 at the Wayback Machine." Biological Journal of the Linnean Society 87.2 (2006): 185-193. doi:10.1111/j.1095-8312.2006.00566.x
- ^ DIXSON33, Alan, N. YHOL T. Jenna, and Matt Anderson. "A positive relationship between baculum length and prolonged intromission patterns in mammals." 动物学报 50.4 (2004): 490-503.
- ^ Norris, David O.; Lopez, Kristin H. (2024-08-08). Hormones and Reproduction of Vertebrates, Volume 5: Mammals. Elsevier. ISBN 978-0-443-15985-5.
- ^ Lombardi, Julian (2012-12-06). Comparative Vertebrate Reproduction. Springer Science & Business Media. ISBN 978-1-4615-4937-6.
- ^ Dixson, Alan F. (2012-01-26). Primate Sexuality: Comparative Studies of the Prosimians, Monkeys, Apes, and Humans. OUP Oxford. ISBN 978-0-19-954464-6.
- ^ Encyclopedia of Behavioral Neuroscience. Elsevier. 2010-06-03. ISBN 978-0-08-045396-5.
- ^ Jöchle, Wolfgang (1973). "Coitus-induced ovulation". Contraception. 7 (6): 523–564. doi:10.1016/0010-7824(73)90023-1. ISSN 0010-7824.