Levmetamfetamine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Vicks VapoInhaler, Everclear Inhaler, others |
| Other names | Levomethamphetamine; Levodesoxyephedrine |
| Routes of administration | Medical: Intranasal Recreational: By mouth, intravenous, insufflation, inhalation, suppository |
| Drug class | Norepinephrine releasing agent; Sympathomimetic; Decongestant |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Oral: ~100%[2][3] |
| Metabolism | Liver (CYP2D6)[5][6] |
| Metabolites | Levoamphetamine[2][4][3] |
| Elimination half-life | 10–15 hours[2][4][3] |
| Excretion | Urine (41–49% unchanged, 2–3% as levoamphetamine)[2][4][3] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.046.974 |
| Chemical and physical data | |
| Formula | C10H15N |
| Molar mass | 149.237 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Levorotatory enantiomer |
| |
| |
| (verify) | |
Levmetamfetamine, also known as l-desoxyephedrine or levomethamphetamine, and commonly sold under the brand name Vicks VapoInhaler among others, is an optical isomer of methamphetamine primarily used as a topical nasal decongestant.[2] It is used to treat nasal congestion from allergies and the common cold.[7] It was first used medically as decongestant beginning in 1958 and has been used for such purposes, primarily in the United States, since then.[8]
Medical uses
[edit]Levmetamfetamine is used to treat nasal congestion related to the common cold and allergic rhinitis. It is available in the form of an inhaler containing 50 mg total per inhaler and delivering between 0.04 and 0.15 mg of the drug per inhalation.[2] Inhalers with a total of 113 mg levmetamfetamine were previously marketed in the United States, but the total amount was eventually reduced to 50 mg.[2]
Side effects
[edit]When the nasal decongestant is taken in excess, levmetamfetamine has potential side effects. These would be similar to those of other decongestants.
Pharmacology
[edit]Pharmacodynamics
[edit]| Compound | NE | DA | 5-HT | Ref | ||
|---|---|---|---|---|---|---|
| Phenethylamine | 10.9 | 39.5 | >10,000 | [9][10][11] | ||
| Amphetamine | ND | ND | ND | ND | ||
| D-Amphetamine | 6.6–7.2 | 5.8–24.8 | 698–1,765 | [12][13] | ||
| L-Amphetamine | 9.5 | 27.7 | ND | [10][11] | ||
| Racephedrine | ND | ND | ND | ND | ||
| Ephedrine (D-) | 43.1–72.4 | 236–1,350 | >10,000 | [12] | ||
| L-Ephedrine | 218 | 2,104 | >10,000 | [12][14] | ||
| Methamphetamine | ND | ND | ND | ND | ||
| D-Methamphetamine | 12.3–13.8 | 8.5–24.5 | 736–1,292 | [12][15] | ||
| L-Methamphetamine | 28.5 | 416 | 4,640 | [12] | ||
| Racemic pseudoephedrine | ND | ND | ND | ND | ||
| D-Pseudoephedrine | 4,092 | 9,125 | >10,000 | [14] | ||
| Pseudoephedrine (L-) | 224 | 1,988 | >10,000 | [14] | ||
| Notes: The smaller the value, the more strongly the drug releases the neurotransmitter. See also Monoamine releasing agent § Activity profiles for a larger table with more compounds. Refs: [16][17] | ||||||
Levmetamfetamine acts as a selective norepinephrine releasing agent.[12][16][18][4] The potencies of levmetamfetamine, levoamphetamine, dextromethamphetamine, and dextroamphetamine in terms of norepinephrine release in vitro and in vivo in rats are all similar.[19][20][21][22][16]
Conversely, whereas dextromethamphetamine and dextroamphetamine are relatively balanced releasers of dopamine and norepinephrine in vitro, levmetamfetamine is about 15- to 20-fold less potent in inducing dopamine release relative to norepinephrine release.[16][18][4][12][21] Moreover, whereas levoamphetamine is about 3- to 5-fold less potent in terms of dopamine release than dextroamphetamine in vivo, levmetamfetamine is dramatically less potent than dextromethamphetamine and substantially less potent than levoamphetamine in this regard.[20][19][22]
In accordance with the findings of catecholamine release studies, levmetamfetamine is 2- to 10-fold or more less potent than dextromethamphetamine in terms of psychostimulant-like effects in rodents.[23][24][25] For comparison, levoamphetamine is only 1- to 4-fold less potent than dextroamphetamine in its stimulating and reinforcing effects in monkeys and humans.[19][26]
The effects of levmetamfetamine are qualitatively distinct relative to those of racemic methamphetamine and dextromethamphetamine and it does not possess the same potential for euphoria or addiction that these drugs possesses.[2][25][27][4][22] In clinical studies, levmetamfetamine at oral doses of 1 to 10 mg has been found not to affect subjective drug responses, heart rate, blood pressure, core temperature, electrocardiography, respiration rate, oxygen saturation, or other clinical parameters.[2][3] As such, doses of levmetamfetamine of less than or equal to 10 mg have no significant physiological or subjective effects.[2][3] However, higher doses of levmetamfetamine, for instance 0.25 to 0.5 mg/kg (mean doses of ~18–37 mg) intravenously, have been reported to produce significant pharmacological effects, including increased heart rate and blood pressure, increased respiration rate, and subjective effects like intoxication and drug liking.[2][4] On the other hand, in contrast to dextromethamphetamine, levmetamfetamine also produces subjective "bad" or aversive drug effects.[18][4] Among the physiological effects of levmetamfetamine is vasoconstriction, which makes it useful for nasal decongestion.[28]
For comparison to levmetamfetamine, 5 to 60 mg oral doses of the related drug levoamphetamine have been used clinically and have been reported to produce significant pharmacological effects, for instance on wakefulness and mood.[29][30][31][26][note 1]
In addition to its norepinephrine-releasing activity, levmetamfetamine is also an agonist of the trace amine-associated receptor 1 (TAAR1).[32][33][34] Levmetamfetamine has also been found to act as a catecholaminergic activity enhancer (CAE), notably at much lower concentrations than its catecholamine releasing activity.[35][36][37][38][39] It is 1- to 10-fold less potent than selegiline but is 3- to 5-fold more potent than dextromethamphetamine in this action.[36][37][38] The CAE effects of such agents may be mediated by TAAR1 agonism.[40][39]
Pharmacokinetics
[edit]Absorption
[edit]The bioavailability of levmetamfetamine is approximately 100%.[2][3] The peak levels of levmetamfetamine range from 3.3 to 31.4 ng/mL with single oral doses of 1 to 10 mg and from 65.4 to 125.9 ng/mL with single intravenous doses of 0.25 to 0.5 mg/kg.[2][4][41] The area-under-the-curve (AUC) levels of levmetamfetamine range from 73.0 to 694.7 ng⋅h/mL with single oral doses of 1 to 10 mg and from 1,190.7 to 2,368.1 mg/kg with single intravenous doses of 0.25 to 0.5 mg/kg.[2][4][41]
Distribution
[edit]The volume of distribution of levmetamfetamine is 288.5 to 315.5 L or 4.15 to 4.17 L/kg.[2][4][3]
Metabolism
[edit]The pharmacokinetics of levmetamfetamine generated as a metabolite from selegiline have been found to be significantly different in CYP2D6 poor metabolizers versus extensive metabolizers.[5][6] Area-under-the-curve (AUC) levels of levmetamfetamine were 46% higher and its elimination half-life was 33% longer in CYP2D6 poor metabolizers compared to extensive metabolizers.[5][6] These findings suggest that CYP2D6 may be significantly involved in the metabolism of levmetamfetamine.[5][6]
Levmetamfetamine is metabolized into levoamphetamine in small amounts.[2][4][3]
Elimination
[edit]Levmetamfetamine is excreted in urine 40.8 to 49.0% as unchanged levmetamfetamine and 2.1 to 3.3% as levoamphetamine.[2][4][3]
The mean elimination half-life of levmetamfetamine ranges between 10.2 and 15.0 hours.[2][4] For comparison, the elimination half-life of dextromethamphetamine was around 10.2 to 10.7 hours in the same studies.[2][4] The clearance of levmetamfetamine is 15.5 to 19.1 L/h or 0.221 L/h⋅kg.[2][4][3]
With selegiline at an oral dose of 10 mg, levmetamfetamine and levoamphetamine are eliminated in urine and recovery of levmetamfetamine is 20 to 60% (or about 2–6 mg) while that of levoamphetamine is 9 to 30% (or about 1–3 mg).[42]
Chemistry
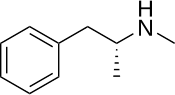
[edit]Levmetamfetamine, also known as L-α,N-dimethyl-β-phenylethylamine or as L-N-methylamphetamine, is a substituted phenethylamine and amphetamine.[2][43] It is the levorotatory enantiomer of methamphetamine.[2] Racemic methamphetamine contains two optical isomers in equal amounts, dextromethamphetamine (the dextrorotatory enantiomer) and levmetamfetamine.[2]
Detection in body fluids
[edit]Levmetamfetamine can register on urine drug tests as either methamphetamine, amphetamine, or both, depending on the subject's metabolism and dosage. Levmetamfetamine metabolizes completely into levoamphetamine after a period of time.[44]
History
[edit]Methamphetamine, a racemic mixture of dextromethamphetamine and levmetamfetamine, was first discovered and synthesized in 1919.[45][46] Methamphetamine was first introduced for medical use in 1938 in oral form under the brand name Pervitin in Germany.[45][46] Over-the-counter nasal decongestant inhalers containing enantiopure levmetamfetamine, originally labeled with the chemical name l-desoxyephedrine, were first introduced in 1958 under the brand name Vicks Inhaler.[8][47][48] By 1995, the brand name was changed to Vicks Vapor Inhaler.[49][50] In 1998, the United States Food and Drug Administration (FDA) required that the chemical name on the labeling be changed from l-desoxyephedrine to levmetamfetamine.[51]
Society and culture
[edit]Recreational use
[edit]As of 2006, there were no studies demonstrating "drug liking" scores of oral levmetamfetamine that are similar to racemic methamphetamine or dextromethamphetamine in either recreational users or medicinal users.[4] In any case, misuse of levmetamfetamine at high doses has been reported.[52][53][54][55]
In recent years, tighter controls in Mexico on certain methamphetamine precursors like ephedrine and pseudoephedrine has led to a greater percentage of illicit methamphetamine from Mexican drug cartels consisting of a higher ratio of levmetamfetamine to dextromethamphetamine within batches of racemic methamphetamine.[56]
Manufacturing
[edit]The manufacturing of levmetamfetamine products for therapeutic use is done according to government regulations and pharmacopeia monographs. The most recent change in Food and Drug Administration regulations for levmetamfetamine inhalers was in 1994, with the adoption of a final monograph.[57]
Notes
[edit]References
[edit]- ^ Anvisa (28 May 2024). "RDC Nº 877 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 877 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União. Archived from the original on 25 September 2024. Retrieved 25 September 2024.
- ^ a b c d e f g h i j k l m n o p q r s t u v w Barkholtz HM, Hadzima R, Miles A (July 2023). "Pharmacology of R-(-)-Methamphetamine in Humans: A Systematic Review of the Literature". ACS Pharmacol Transl Sci. 6 (7): 914–924. doi:10.1021/acsptsci.3c00019. PMC 10353062. PMID 37470013.
- ^ a b c d e f g h i j k Li L, Lopez JC, Galloway GP, Baggott MJ, Everhart T, Mendelson J (August 2010). "Estimating the intake of abused methamphetamines using experimenter-administered deuterium labeled R-methamphetamine: selection of the R-methamphetamine dose". Ther Drug Monit. 32 (4): 504–7. doi:10.1097/FTD.0b013e3181db82f2. PMC 3040572. PMID 20592647.
- ^ a b c d e f g h i j k l m n o p q Mendelson J, Uemura N, Harris D, Nath RP, Fernandez E, Jacob P, et al. (October 2006). "Human pharmacology of the methamphetamine stereoisomers". Clin Pharmacol Ther. 80 (4): 403–420. doi:10.1016/j.clpt.2006.06.013. PMID 17015058.
The stereoisomers of methamphetamine produce markedly different dopamine, norepinephrine, and serotonin responses in various brain regions in rats.41,42 d-Methamphetamine (2 mg/kg) is more potent in releasing caudate dopamine than l-methamphetamine (12 and 18 mg/kg). By use of in vitro uptake and release assays, d-methamphetamine (50% effective concentration [EC50], 24.5 ± 2.1 nmol/L) was 17 times more potent in releasing dopamine than l-methamphetamine (EC50, 416 ± 20 nmol/L) and significantly more potent in blocking dopamine uptake (inhibition constant [Ki ], 114 ± 11 nm versus 4840 ± 178 nm).12,13
- ^ a b c d Kraemer T, Maurer HH (April 2002). "Toxicokinetics of amphetamines: metabolism and toxicokinetic data of designer drugs, amphetamine, methamphetamine, and their N-alkyl derivatives". Ther Drug Monit. 24 (2): 277–89. doi:10.1097/00007691-200204000-00009. PMID 11897973.
- ^ a b c d Scheinin H, Anttila M, Dahl ML, Karnani H, Nyman L, Taavitsainen P, et al. (October 1998). "CYP2D6 polymorphism is not crucial for the disposition of selegiline". Clin Pharmacol Ther. 64 (4): 402–411. doi:10.1016/S0009-9236(98)90071-6. PMID 9797797.
- ^ "Cold, Cough, Allergy, Bronchodilator, and Antiasthmatic Drug Products for Over-the-Counter Human Use". FDA. 7 June 2023. Archived from the original on 6 July 2023.
- ^ a b Wesson DR, Smith DE, Morgan JP (1986). "The international scheduling of OTC inhaler ingredients: an abuse perspective". J Psychoactive Drugs. 18 (2): 151–4. doi:10.1080/02791072.1986.10471394 (inactive 11 November 2024). PMID 2874202.
The Vicks® Inhaler initially contained only aromatics, but in 1958. Vicks added l-methamphetamine. The package labeling used the alternative chemical term, l-desoxyephedrine. Thus, prior to 1958, it contained nothing psychoactive and had no association with drug abuse.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ Reith ME, Blough BE, Hong WC, Jones KT, Schmitt KC, Baumann MH, et al. (February 2015). "Behavioral, biological, and chemical perspectives on atypical agents targeting the dopamine transporter". Drug and Alcohol Dependence. 147: 1–19. doi:10.1016/j.drugalcdep.2014.12.005. PMC 4297708. PMID 25548026.
- ^ a b Forsyth AN (22 May 2012). "Synthesis and Biological Evaluation of Rigid Analogues of Methamphetamines". ScholarWorks@UNO. Retrieved 4 November 2024.
- ^ a b Blough B (July 2008). "Dopamine-releasing agents" (PDF). In Trudell ML, Izenwasser S (eds.). Dopamine Transporters: Chemistry, Biology and Pharmacology. Hoboken [NJ]: Wiley. pp. 305–320. ISBN 978-0-470-11790-3. OCLC 181862653. OL 18589888W.
- ^ a b c d e f g Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, et al. (January 2001). "Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin". Synapse. 39 (1): 32–41. doi:10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. PMID 11071707.
- ^ Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, et al. (2013). "Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive 'bath salts' products". Neuropsychopharmacology. 38 (4): 552–562. doi:10.1038/npp.2012.204. PMC 3572453. PMID 23072836.
- ^ a b c Rothman RB, Vu N, Partilla JS, Roth BL, Hufeisen SJ, Compton-Toth BA, et al. (2003). "In vitro characterization of ephedrine-related stereoisomers at biogenic amine transporters and the receptorome reveals selective actions as norepinephrine transporter substrates". J. Pharmacol. Exp. Ther. 307 (1): 138–45. doi:10.1124/jpet.103.053975. PMID 12954796. S2CID 19015584.
- ^ Baumann MH, Ayestas MA, Partilla JS, Sink JR, Shulgin AT, Daley PF, et al. (2012). "The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue". Neuropsychopharmacology. 37 (5): 1192–1203. doi:10.1038/npp.2011.304. PMC 3306880. PMID 22169943.
- ^ a b c d Rothman RB, Baumann MH (October 2003). "Monoamine transporters and psychostimulant drugs". Eur J Pharmacol. 479 (1–3): 23–40. doi:10.1016/j.ejphar.2003.08.054. PMID 14612135.
- ^ Rothman RB, Baumann MH (2006). "Therapeutic potential of monoamine transporter substrates". Current Topics in Medicinal Chemistry. 6 (17): 1845–1859. doi:10.2174/156802606778249766. PMID 17017961.
- ^ a b c Kohut SJ, Jacobs DS, Rothman RB, Partilla JS, Bergman J, Blough BE (December 2017). "Cocaine-like discriminative stimulus effects of "norepinephrine-preferring" monoamine releasers: time course and interaction studies in rhesus monkeys". Psychopharmacology (Berl). 234 (23–24): 3455–3465. doi:10.1007/s00213-017-4731-5. PMC 5747253. PMID 28889212.
In the present experiments, two monoamine releasers, l-MA and PAL-329, were shown to produce cocaine-like discriminative-stimulus effects in monkeys, suggesting that they meet the above criteria. One of these compounds, l-MA, also has been shown to serve as a positive reinforcer in rodents (Yokel and Pickens 1973) and monkeys (Winger et al 1994), further confirming the overlap with behavioral effects of cocaine. Both compounds also exhibit an approximately 15-fold greater potency in releasing NE than DA, which may be therapeutically advantageous. For example, the subjective effects of l-MA in human studies are similar in some respects to those of d-MA. However, the subjective effects of the two isomers also differ in potentially important ways. While both l-MA and d-MA produce subjective ratings of "drug liking" and "good effects" in experienced stimulant users, only lMA produces concomitant ratings of bad or aversive drug effects (Mendelson et al 2006), a factor which may limit its abuse liability.
- ^ a b c Heal DJ, Smith SL, Gosden J, Nutt DJ (June 2013). "Amphetamine, past and present--a pharmacological and clinical perspective". J Psychopharmacol. 27 (6): 479–496. doi:10.1177/0269881113482532. PMC 3666194. PMID 23539642.
- ^ a b Nishino S, Kotorii N (2016). "Modes of Action of Drugs Related to Narcolepsy: Pharmacology of Wake-Promoting Compounds and Anticataplectics". Narcolepsy: A Clinical Guide (2nd ed.). Cham: Springer International Publishing. pp. 307–329. doi:10.1007/978-3-319-23739-8_22. ISBN 978-3-319-23738-1.
- ^ a b Xue Z, Siemian JN, Zhu Q, Blough BE, Li JX (August 2019). "Further pharmacological comparison of D-methamphetamine and L-methamphetamine in rats: abuse-related behavioral and physiological indices". Behav Pharmacol. 30 (5): 422–428. doi:10.1097/FBP.0000000000000453. PMC 6529304. PMID 30480551.
When considered with neurochemical data that l-MA is similarly potent in releasing norepinephrine (NE) but 15- to 20-fold less potent in releasing dopamine (DA), as compared to d-MA (Kuczenski et al., 1995; Melega et al., 1999), l-MA may appear to carry lower abuse liability than d-MA.
- ^ a b c Kuczenski R, Segal DS, Cho AK, Melega W (February 1995). "Hippocampus norepinephrine, caudate dopamine and serotonin, and behavioral responses to the stereoisomers of amphetamine and methamphetamine". The Journal of Neuroscience. 15 (2): 1308–1317. doi:10.1523/jneurosci.15-02-01308.1995. PMC 6577819. PMID 7869099.
Consistent with our past results, in response to 2 mg/kg D-AMPH, mean caudate extracellular DA increased approximately 15-fold to a peak concentration of 688 ± 121 nM during the initial 20 min interval, then returned to baseline over the next 3 hr. Similarly, in response to 2 mg/kg D-METH, DA increased to a peak concentration of 648 ± 71 nM during the initial 20 min interval and then declined toward baseline. In contrast, in response to both 6 mg/kg L-AMPH and 12 mg/kg L-METH, peak DA concentrations (508 ± 51 and 287 ± 49 nM, respectively) were delayed to the second 20 min interval, before returning toward baseline. [...] Similar to our previous results, 2 mg/kg D-AMPH increased NE to a maximum of 29.3 ± 3.1 nM, about 20-fold over baseline, during the second 20 min interval. L-AMPH (6 mg/kg) produced a comparable effect, increasing NE concentrations to 32.0 ± 8.9 nM. In contrast, D-METH promoted an increase in NE to 12.0 ± 1.2 nM which was significantly lower than all other groups, whereas L-METH promoted an increase to 64.8 ± 4.9 nM, which was significantly higher than all other groups.
- ^ Nishimura T, Takahata K, Kosugi Y, Tanabe T, Muraoka S (May 2017). "Psychomotor effect differences between l-methamphetamine and d-methamphetamine are independent of murine plasma and brain pharmacokinetics profiles". J Neural Transm (Vienna). 124 (5): 519–523. doi:10.1007/s00702-017-1694-y. PMC 5399046. PMID 28213761.
There have been no studies directly comparing the pharmacodynamics and pharmacokinetics of the methamphetamine enantiomers in mice. It is often suggested that dmethamphetamine exerts more potent physiological and pharmacological effects than l-methamphetamine does, and that the stimulating effects exerted by l-methamphetamine on the central nervous system are 2–10 times less potent than those of d-methamphetamine (Mendelson et al. 2006). The results of the present study indicated that psychostimulant effects induced by l-methamphetamine are lower than those elicited by one-tenth the dose of d-methamphetamine. In addition, plasma pharmacokinetic parameters and striatal concentrations of methamphetamine following administration of l-methamphetamine at 10 mg/ kg (which did not induce psychomotor activity) were approximately 11 and 16 times as high, respectively, as those following administration of 1 mg/kg d-methamphetamine. Despite the fact that there are differentiable psycho-stimulating effects between two enantiomers, no significant difference in plasma pharmacokinetic parameters was detected at 1 mg/kg.
- ^ Siemian JN, Xue Z, Blough BE, Li JX (July 2017). "Comparison of some behavioral effects of d- and l-methamphetamine in adult male rats". Psychopharmacology (Berl). 234 (14): 2167–2176. doi:10.1007/s00213-017-4623-8. PMC 5482751. PMID 28386698.
- ^ a b Pauly RC, Bhimani RV, Li JX, Blough BE, Landavazo A, Park J (March 2023). "Distinct Effects of Methamphetamine Isomers on Limbic Norepinephrine and Dopamine Transmission in the Rat Brain". ACS Chemical Neuroscience: acschemneuro.2c00689. doi:10.1021/acschemneuro.2c00689. PMID 36976755. S2CID 257772503.
- ^ a b c d Smith RC, Davis JM (June 1977). "Comparative effects of d-amphetamine, l-amphetamine, and methylphenidate on mood in man". Psychopharmacology (Berl). 53 (1): 1–12. doi:10.1007/BF00426687. PMID 407607.
[...] the 2:1 ratio of d- and l-AMP effects on euphoric mood is very similar to the ratios (1.3:1 to 2.1:1) which have been reported for the efficacy of amphetamine isomers on other classes of behavior in man—for example, the activation of psychosis and the treatment of hyperkinetic children (see Table 1). [...] Table 1. Some previous studies comparing effects of d-amphetamine, l-amphetamine, and methylphenidate in man. [...]
- ^ Melega WP, Cho AK, Schmitz D, Kuczenski R, Segal DS (February 1999). "l-methamphetamine pharmacokinetics and pharmacodynamics for assessment of in vivo deprenyl-derived l-methamphetamine". The Journal of Pharmacology and Experimental Therapeutics. 288 (2): 752–758. PMID 9918585.
- ^ Pray SW (19 February 2010). "Nonprescription Products to Avoid With Hypertension". uspharmacist.com. Archived from the original on 30 October 2014. Retrieved 17 October 2014.
Topical Nasal Decongestants: Most topical nasal decongestants also carry the warning against unsupervised use with hypertension. This includes oxymetazoline (e.g., Afrin), phenylephrine (e.g., Neo-Synephrine), naphazoline (e.g., Privine), and l-desoxyephedrine/levomethamphetamine. When hypertensive patients request a nasal decongestant, the pharmacist can recommend several alternatives. Propylhexedrine (e.g., Benzedrex Inhaler) is not required to carry a warning against unsupervised use with hypertension and may be effective. Another option is the nasal strip (e.g., Breathe Right). When properly applied, the strip can open the nostrils slightly, and perhaps sufficiently to allow the patient to breathe without use of a pharmacologically active ingredient.
- ^ Silverstone T, Wells B (1980). "Clinical Psychopharmacology of Amphetamine and Related Compounds". Amphetamines and Related Stimulants: Chemical, Biological, Clinical, and Sociological Aspects. CRC Press. pp. 147–160. doi:10.1201/9780429279843-10. ISBN 978-0-429-27984-3.
A comparison of dextroamphetamine and levoamphetamine revealed that the dextrorotatory isomer was the more potent in elevating mood in normal subjects, being at least twice as potent as the levo form.35 [...] Narcolepsy was one of the first conditions to be treated successfully with amphetamine3 and remains one of the few (some would say the only) clinical indications for its use. While the required oral dose of dextroamphetamine (Dexedrine®) ranges from 5 to 120 mg/day, most patients respond to 10 mg two to four times daily. [...] The closely related compound methylphenidate (Ritalin®), 20 mg two to four times daily, has been shown to be as effective as dextroamphetamine but with less likelihood of causing side effects.61 The same is true of levoamphetamine.62 [...] Nevertheless, as amphetamine has an action on dopaminergic pathways it was considered worthwhile to examine the effects of amphetamine under controlled conditions.95 Twenty patients, all on other anti-Parkinsonian drugs, were studied. There was some subjective improvement in a proportion (less than half) of the patients when they received either dextroamphetamine or levoamphetamine, but there was little objective improvement. The authors remarked that amphetamine was unlikely to have worked anyway in Parkinson's disease as it acts mainly by releasing dopamine and noradrenaline from presynaptic neurons; as the underlying pathology involves a reduction of presynaptic dopamine, there would be insufficient dopamine for amphetamine to release.
- ^ Parkes JD, Fenton GW (December 1973). "Levo(-) amphetamine and dextro(+) amphetamine in the treatment of narcolepsy". J Neurol Neurosurg Psychiatry. 36 (6): 1076–81. doi:10.1136/jnnp.36.6.1076. PMC 1083612. PMID 4359162.
- ^ Parkes JD, Tarsy D, Marsden CD, Bovill KT, Phipps JA, Rose P, et al. (March 1975). "Amphetamines in the treatment of Parkinson's disease". J Neurol Neurosurg Psychiatry. 38 (3): 232–7. doi:10.1136/jnnp.38.3.232. PMC 491901. PMID 1097600.
- ^ "Levmetamfetamine". PubChem. National Center for Biotechnology Information, U.S. National Library of Medicine. Archived from the original on 18 October 2014. Retrieved 17 October 2014.
- ^ Sotnikova TD, Caron MG, Gainetdinov RR (August 2009). "Trace amine-associated receptors as emerging therapeutic targets". Mol Pharmacol. 76 (2): 229–35. doi:10.1124/mol.109.055970. PMC 2713119. PMID 19389919.
Intriguingly, d- and l-amphetamine, methamphetamine, 3,4-methylenedioxymethamphetamine (MDMA), and other closely related compounds are also able to activate TAAR1 receptors in vitro as evidenced by cAMP stimulation in human embryonic kidney cells.
- ^ Reese EA, Norimatsu Y, Grandy MS, Suchland KL, Bunzow JR, Grandy DK (January 2014). "Exploring the determinants of trace amine-associated receptor 1's functional selectivity for the stereoisomers of amphetamine and methamphetamine". J Med Chem. 57 (2): 378–390. doi:10.1021/jm401316v. PMID 24354319.
- ^ Knoll J (2001). "Antiaging compounds: (-)deprenyl (selegeline) and (-)1-(benzofuran-2-yl)-2-propylaminopentane, [(-)BPAP], a selective highly potent enhancer of the impulse propagation mediated release of catecholamine and serotonin in the brain". CNS Drug Rev. 7 (3): 317–45. doi:10.1111/j.1527-3458.2001.tb00202.x. PMC 6494119. PMID 11607046.
- ^ a b Knoll J (February 1998). "(-)Deprenyl (selegiline), a catecholaminergic activity enhancer (CAE) substance acting in the brain". Pharmacol Toxicol. 82 (2): 57–66. doi:10.1111/j.1600-0773.1998.tb01399.x. PMID 9498233.
- ^ a b Knoll J, Miklya I, Knoll B, Markó R, Kelemen K (1996). "(-)Deprenyl and (-)1-phenyl-2-propylaminopentane, [(-)PPAP], act primarily as potent stimulants of action potential-transmitter release coupling in the catecholaminergic neurons". Life Sci. 58 (10): 817–827. doi:10.1016/0024-3205(96)00014-8. PMID 8602114.
- ^ a b Knoll J, Miklya I (1994). "Multiple, small dose administration of (-)deprenyl enhances catecholaminergic activity and diminishes serotoninergic activity in the brain and these effects are unrelated to MAO-B inhibition". Arch Int Pharmacodyn Ther. 328 (1): 1–15. PMID 7893186.
- ^ a b Harsing LG, Timar J, Miklya I (August 2023). "Striking Neurochemical and Behavioral Differences in the Mode of Action of Selegiline and Rasagiline". Int J Mol Sci. 24 (17): 13334. doi:10.3390/ijms241713334. PMC 10487936. PMID 37686140.
- ^ Harsing LG, Knoll J, Miklya I (August 2022). "Enhancer Regulation of Dopaminergic Neurochemical Transmission in the Striatum". Int J Mol Sci. 23 (15): 8543. doi:10.3390/ijms23158543. PMC 9369307. PMID 35955676.
- ^ a b Li L, Everhart T, Jacob Iii P, Jones R, Mendelson J (February 2010). "Stereoselectivity in the human metabolism of methamphetamine". Br J Clin Pharmacol. 69 (2): 187–192. doi:10.1111/j.1365-2125.2009.03576.x. PMC 2824480. PMID 20233182.
- ^ Heinonen EH, Lammintausta R (1991). "A review of the pharmacology of selegiline". Acta Neurol Scand Suppl. 136: 44–59. doi:10.1111/j.1600-0404.1991.tb05020.x. PMID 1686954.
- ^ "Levmetamfetamine". PubChem. Retrieved 31 July 2024.
- ^ DeGeorge M, Weber J (30 November 2012). "Methamphetamine Urine Toxicology: An In-depth Review". Practical Pain Management. Vertical Health LLC. Archived from the original on 13 February 2016. Retrieved 21 February 2016.
- ^ a b Vearrier D, Greenberg MI, Miller SN, Okaneku JT, Haggerty DA (February 2012). "Methamphetamine: history, pathophysiology, adverse health effects, current trends, and hazards associated with the clandestine manufacture of methamphetamine". Disease-a-Month. 58 (2): 38–89. doi:10.1016/j.disamonth.2011.09.004. PMID 22251899.
Japanese chemist Akira Ogata first synthesized methamphetamine in 1919 using ephedrine as a precursor. [...] In 1959 the S. Pfeiffer Company began producing Valo inhalers that contained 150-200 mg of methamphetamine.4,5 [...] Temmler Pharmaceutical Company introduced Pervitin in 1938 to the European market. Pervitin was available as 3 mg tablets that physicians could provide for the German military units.
- ^ a b Ciccarone D (March 2011). "Stimulant abuse: pharmacology, cocaine, methamphetamine, treatment, attempts at pharmacotherapy". Prim Care. 38 (1): 41–58. doi:10.1016/j.pop.2010.11.004. PMC 3056348. PMID 21356420.
In 1919, Japanese chemist Akira Ogata, as part of his effort to prove the structure of ephedrine, reported the synthesis of the closely related compound we now call methamphetamine, and this result was described in the Western literature (Amatsu & Kubota, 1913; Lee, 2011; Ogata, 1920). [...] As a result, when competitors began to consider emulating SKF's success in the late 1930s, they turned to methamphetamine, which had nearly indistinguishable effects but—because its synthesis together with its pharmacological characteristics was published before 1920—was free from patent encumbrance. [...] In any event, by 1940 Benzedrine Sulfate had achieved medical acclaim and quickly growing sales as an antidepressant effective for milder forms of the condition, both in the United States and the United Kingdom. In Germany, the Temmler drug firm quickly copied SKF, marketing methamphetamine (again, unprotected by patents) tablets under the Pervitin brand, with claims that it restored "joy in work" in cases of mild depression around 1938 (Rasmussen, 2006; Steinkamp, 2006).
- ^ Di Cyan E, Hessman L (1972). Without Prescription: A Guide to the Selection and Use of Medicines You Can Get Over-the-counter Without Prescription, for Safe Self-medication. A Fireside book. Simon and Schuster. p. 53. ISBN 978-0-671-21137-0. Retrieved 9 July 2024.
Vicks Inhaler (Vick)—Contains l-desoxyephedrine, [menthol], camphor, methyl salicylate, and bromyl acetate.
- ^ Krantz JC, Carr CJ, Aviado DM (1972). Krantz and Carr's Pharmacologic Principles of Medical Practice: A Textbook on Pharmacology and Therapeutics for Students and Practitioners of Medicine, Pharmacy, and Dentistry. Williams & Wilkins. p. 389. ISBN 978-0-683-00292-8. Retrieved 9 July 2024.
Methamphetamine, also known as desoxyephedrine, is available as an inhalant (VICKS INHALER). The volatile base of methamphetamine is mixed with menthol, camphor, methyl salicylate, oil of sassafras and bornylacetate, which add to the customer acceptibility of the inhalant. The nasal decongestant effect of methamphetamine has been demonstrated in the experimental animal (Aviado et al., 1959). The other pharmacologic features of methamphetamine are discussed under its use as a vasopressor drug (Section VIII) and an anorexigenic drug (Section XV).
- ^ American Pharmaceutical Association (1995). Handbook of Non-prescription Drugs. American Pharmaceutical Association. p. 109. ISBN 978-0-917330-70-4. Retrieved 9 July 2024.
Product & Manufacturer or Supplier: Vicks Vapor Inhaler, Procter & Gamble. Dosage Form: nasal inhaler. Sympathomimetic Agent: levodesoxyephedrine, 50 mg/inhaler. Preservative: None. Other Ingredients: bornyl acetate • camphor • lavender oil • menthol.
- ^ Rapp R (1997). The Pill Book Guide. Bantam Books. p. 220. ISBN 978-0-553-57729-7. Retrieved 9 July 2024.
Vicks Vapor Inhaler (VIKS): Generic Ingredient: l-Desoxyephedrine. Type of Drug: Topical decongestant. Used for: Temporary relief of nasal congestion due to colds and allergies. General Information: Vicks Vapor Inhaler contains l-desoxyephedrine, which acts as a topical decongestant by narrowing or constricting blood vessels in the nose. This action reduces the blood supplied to the nose and decreases the swelling of nasal mucous membranes. [...]
- ^ Bovett R (January 2006). "Meth Epidemic Solutions". North Dakota Law Review. 82 (4): 1195–1216.
Rules & Regulations Dep't of Health & Human Services, 61 Fed. Reg. 9,570 (Mar. 8, 1996) (codified at 21 C.F.R pt. 321). Vicks® Vapor Inhaler uses this active ingredient. For a time, the active ingredient was labeled "l-desoxyephedrine," which is simply another name for lmeth. Id. The FDA later changed the labeling requirement to "levmetamfetamine." Rules & Regulations Dep't of Health & Human Services, 63 Fed. Reg. 40,647 (July 30, 1998) (codified at 21 C.F.R. pts. 310 and 321).
- ^ Mendelson JE, McGlothlin D, Harris DS, Foster E, Everhart T, Jacob P, et al. (July 2008). "The clinical pharmacology of intranasal l-methamphetamine". BMC Clin Pharmacol. 8: 4. doi:10.1186/1472-6904-8-4. PMC 2496900. PMID 18644153.
The 64-inhalation condition produced a small (change score of ~6) increase in "Good Drug Effect" suggesting a low potential for abuse even though occurrences of inhaler abuse is reported in the literature [1,18,19]. Larger doses of intravenous lmethamphetamine are psychoactive and may have some abuse potential in methamphetamine users [16].
- ^ Gal J (1982). "Amphetamines in Nasal Inhalers". Journal of Toxicology: Clinical Toxicology. 19 (5): 517–518. doi:10.3109/15563658208992508. ISSN 0731-3810.
- ^ Halle AB, Kessler R, Alvarez M (June 1985). "Drug abuse with Vicks nasal inhaler". South Med J. 78 (6): 761–2. doi:10.1097/00007611-198506000-00043. PMID 4002016.
- ^ Ferrando RL, McCorvey E, Simon WA, Stewart DM (March 1988). "Bizarre behavior following the ingestion of levo-desoxyephedrine". Drug Intell Clin Pharm. 22 (3): 214–217. doi:10.1177/106002808802200308. PMID 3366062.
- ^ Cunningham JK, Maxwell JC, Campollo O, Liu LM, Lattyak WJ, Callaghan RC (April 2013). "Mexico's precursor chemical controls: emergence of less potent types of methamphetamine in the United States". Drug Alcohol Depend. 129 (1–2): 125–36. doi:10.1016/j.drugalcdep.2012.10.001. PMID 23127541.
- ^ Research Cf (28 February 2022). "Rulemaking History for OTC Nasal Decongestant Drug Products". FDA.
