Noble gas compound
In chemistry, noble gas compounds are chemical compounds that include an element from the noble gases, group 18 of the periodic table. Although the noble gases are generally unreactive elements, many such compounds have been observed, particularly involving the element xenon.
From the standpoint of chemistry, the noble gases may be divided into two groups:[citation needed] the relatively reactive krypton (ionisation energy 14.0 eV), xenon (12.1 eV), and radon (10.7 eV) on one side, and the very unreactive argon (15.8 eV), neon (21.6 eV), and helium (24.6 eV) on the other. Consistent with this classification, Kr, Xe, and Rn form compounds that can be isolated in bulk at or near standard temperature and pressure, whereas He, Ne, Ar have been observed to form true chemical bonds using spectroscopic techniques, but only when frozen into a noble gas matrix at temperatures of 40 K (−233 °C; −388 °F) or lower, in supersonic jets of noble gas, or under extremely high pressures with metals.
The heavier noble gases have more electron shells than the lighter ones. Hence, the outermost electrons are subject to a shielding effect from the inner electrons that makes them more easily ionized, since they are less strongly attracted to the positively-charged nucleus. This results in an ionization energy low enough to form stable compounds with the most electronegative elements, fluorine and oxygen, and even with less electronegative elements such as nitrogen and carbon under certain circumstances.[1][2]
History and background
[edit]When the family of noble gases was first identified at the end of the nineteenth century, none of them were observed to form any compounds and so it was initially believed that they were all inert gases (as they were then known) which could not form compounds. With the development of atomic theory in the early twentieth century, their inertness was ascribed to a full valence shell of electrons which render them very chemically stable and nonreactive. All noble gases have full s and p outer electron shells (except helium, which has no p sublevel), and so do not form chemical compounds easily. Their high ionization energy and almost zero electron affinity explain their non-reactivity.
In 1933, Linus Pauling predicted that the heavier noble gases would be able to form compounds with fluorine and oxygen. Specifically, he predicted the existence of krypton hexafluoride (KrF6) and xenon hexafluoride (XeF6), speculated that XeF8 might exist as an unstable compound, and suggested that xenic acid would form perxenate salts.[3][4] These predictions proved quite accurate, although subsequent predictions for XeF8 indicated that it would be not only thermodynamically unstable, but kinetically unstable.[5] As of 2022, XeF8 has not been made, although the octafluoroxenate(VI) anion ([XeF8]2−) has been observed.
By 1960, no compound with a covalently bound noble gas atom had yet been synthesized.[6] The first published report, in June 1962, of a noble gas compound was by Neil Bartlett, who noticed that the highly oxidising compound platinum hexafluoride ionised O2 to O+2. As the ionisation energy of O2 to O+2 (1165 kJ mol−1) is nearly equal to the ionisation energy of Xe to Xe+ (1170 kJ mol−1), he tried the reaction of Xe with PtF6. This yielded a crystalline product, xenon hexafluoroplatinate, whose formula was proposed to be Xe+[PtF6]−.[4][7] It was later shown that the compound is actually more complex, containing both [XeF]+[PtF5]− and [XeF]+[Pt2F11]−.[8] Nonetheless, this was the first real compound of any noble gas.
The first binary noble gas compounds were reported later in 1962. Bartlett synthesized xenon tetrafluoride (XeF4) by subjecting a mixture of xenon and fluorine to high temperature.[9] Rudolf Hoppe, among other groups, synthesized xenon difluoride (XeF2) by the reaction of the elements.[10]
Following the first successful synthesis of xenon compounds, synthesis of krypton difluoride (KrF2) was reported in 1963.[11]
True noble gas compounds
[edit]In this section, the non-radioactive noble gases are considered in decreasing order of atomic weight, which generally reflects the priority of their discovery, and the breadth of available information for these compounds. The radioactive elements radon and oganesson are harder to study and are considered at the end of the section.
Xenon compounds
[edit]After the initial 1962 studies on XeF4 and XeF2, xenon compounds that have been synthesized include other fluorides (XeF6), oxyfluorides (XeOF2, XeOF4, XeO2F2, XeO3F2, XeO2F4) and oxides (XeO2, XeO3 and XeO4). Xenon fluorides react with several other fluorides to form fluoroxenates, such as sodium octafluoroxenate(VI) ((Na+)2[XeF8]2−),[citation needed] and fluoroxenonium salts, such as trifluoroxenonium hexafluoroantimonate ([XeF3]+[SbF6]−).[12]
In terms of other halide reactivity, short-lived excimers of noble gas halides such as XeCl2 or XeCl are prepared in situ, and are used in the function of excimer lasers.[13]
Recently,[when?] xenon has been shown to produce a wide variety of compounds of the type XeOnX2 where n is 1, 2 or 3 and X is any electronegative group, such as CF3, C(SO2CF3)3, N(SO2F)2, N(SO2CF3)2, OTeF5, O(IO2F2), etc.; the range of compounds is impressive, similar to that seen with the neighbouring element iodine, running into the thousands and involving bonds between xenon and oxygen, nitrogen, carbon, boron and even gold, as well as perxenic acid, several halides, and complex ions.[citation needed]
The compound [Xe2]+[Sb4F21]− contains a Xe–Xe bond, which is the longest element-element bond known (308.71 pm = 3.0871 Å).[14] Short-lived excimers of Xe2 are reported to exist as a part of the function of excimer lasers.[citation needed]
Krypton compounds
[edit]Krypton gas reacts with fluorine gas under extreme forcing conditions, forming KrF2 according to the following equation:
- Kr + F2 → KrF2
KrF2 reacts with strong Lewis acids to form salts of the [KrF]+ and [Kr2F3]+ cations.[11] The preparation of KrF4 reported by Grosse in 1963, using the Claasen method, was subsequently shown to be a mistaken identification.[15]
Krypton compounds with other than Kr–F bonds (compounds with atoms other than fluorine) have also been described. KrF2 reacts with B(OTeF5)3 to produce the unstable compound, Kr(OTeF5)2, with a krypton-oxygen bond. A krypton-nitrogen bond is found in the cation [H−C≡N−Kr−F]+, produced by the reaction of KrF2 with [H−C≡N−H]+[AsF6]− below −50 °C.[16]
Argon compounds
[edit]This section needs expansion. You can help by adding to it. (January 2015) |
The discovery of HArF was announced in 2000.[17][18] The compound can exist in low temperature argon matrices for experimental studies, and it has also been studied computationally.[18] Argon hydride ion [ArH]+ was obtained in the 1970s.[19] This molecular ion has also been identified in the Crab nebula, based on the frequency of its light emissions.[20]
There is a possibility that a solid salt of [ArF]+ could be prepared with [SbF6]− or [AuF6]− anions.[21][22]
Neon and helium compounds
[edit]
The ions, Ne+, [NeAr]+, [NeH]+, and [HeNe]+ are known from optical and mass spectrometric studies. Neon also forms an unstable hydrate.[23] There is some empirical and theoretical evidence for a few metastable helium compounds which may exist at very low temperatures or extreme pressures. The stable cation [HeH]+ was reported in 1925,[24] but was not considered a true compound since it is not neutral and cannot be isolated. In 2016 scientists created the helium compound disodium helide (Na2He) which was the first helium compound discovered.[25]
Radon and oganesson compounds
[edit]
Radon is not chemically inert, but its short half-life (3.8 days for 222Rn) and the high energy of its radioactivity make it difficult to investigate its only fluoride (RnF2), its reported oxide (RnO3), and their reaction products.[26]
All known oganesson isotopes have even shorter half-lives in the millisecond range and no compounds are known yet,[27] although some have been predicted theoretically. It is expected to be even more reactive than radon, more like a normal element than a noble gas in its chemistry.[28]
Reports prior to xenon hexafluoroplatinate and xenon tetrafluoride
[edit]Clathrates
[edit]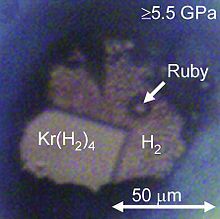
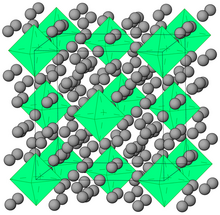
Prior to 1962, the only isolated compounds of noble gases were clathrates (including clathrate hydrates); other compounds such as coordination compounds were observed only by spectroscopic means.[4] Clathrates (also known as cage compounds) are compounds of noble gases in which they are trapped within cavities of crystal lattices of certain organic and inorganic substances. Ar, Kr, Xe and Ne[30] can form clathrates with crystalline hydroquinone. Kr and Xe can appear as guests in crystals of melanophlogite.[31]
Helium-nitrogen (He(N2)11) crystals have been grown at room temperature at pressures ca. 10 GPa in a diamond anvil cell.[32] Solid argon-hydrogen clathrate (Ar(H2)2) has the same crystal structure as the MgZn2 Laves phase. It forms at pressures between 4.3 and 220 GPa, though Raman measurements suggest that the H2 molecules in Ar(H2)2 dissociate above 175 GPa. A similar Kr(H2)4 solid forms at pressures above 5 GPa. It has a face-centered cubic structure where krypton octahedra are surrounded by randomly oriented hydrogen molecules. Meanwhile, in solid Xe(H2)8 xenon atoms form dimers inside solid hydrogen.[29]
Coordination compounds
[edit]Coordination compounds such as Ar·BF3 have been postulated to exist at low temperatures, but have never been confirmed.[citation needed]
Xenon is known to function as a metal ligand. In addition to the charged [AuXe4]2+, xenon, krypton, and argon all reversibly bind to gaseous M(CO)5, where M=Cr, Mo, or W. P-block metals also bind noble gases: XeBeO has been observed spectroscopically and both XeBeS and FXeBO are predicted stable.[33]
Also, compounds such as WHe2 and HgHe2 were reported to have been formed by electron bombardment, but recent research has shown that these are probably the result of He being adsorbed on the surface of the metal; therefore, these compounds cannot truly be considered chemical compounds.[citation needed]
Hydrates
[edit]Hydrates are formed by compressing noble gases in water, where it is believed that the water molecule, a strong dipole, induces a weak dipole in the noble gas atoms, resulting in dipole-dipole interaction. Heavier atoms are more influenced than smaller ones, hence Xe·5.75H2O was reported to have been the most stable hydrate;[34] it has a melting point of 24 °C.[35] The deuterated version of this hydrate has also been produced.[36]
Fullerene adducts
[edit]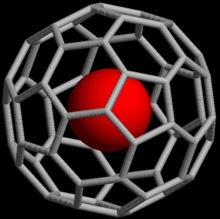
Noble gases can also form endohedral fullerene compounds where the noble gas atom is trapped inside a fullerene molecule. In 1993, it was discovered that when C60 is exposed to a pressure of around 3 bar of He or Ne, the complexes He@C60 and Ne@C60 are formed.[37] Under these conditions, only about one out of every 650,000 C60 cages was doped with a helium atom; with higher pressures (3000 bar), it is possible to achieve a yield of up to 0.1%. Endohedral complexes with argon, krypton and xenon have also been obtained, as well as numerous adducts of He@C60.[38]
Applications
[edit]Most applications of noble gas compounds are either as oxidising agents or as a means to store noble gases in a dense form. Xenic acid is a valuable oxidising agent because it has no potential for introducing impurities—xenon is simply liberated as a gas—and so is rivalled only by ozone in this regard.[4] The perxenates are even more powerful oxidizing agents.[citation needed] Xenon-based oxidants have also been used for synthesizing carbocations stable at room temperature, in SO2ClF solution.[39][non-primary source needed]
Stable salts of xenon containing very high proportions of fluorine by weight (such as tetrafluoroammonium heptafluoroxenate(VI), [NF4][XeF7], and the related tetrafluoroammonium octafluoroxenate(VI) [NF4]2[XeF8]), have been developed as highly energetic oxidisers for use as propellants in rocketry.[40][non-primary source needed] [41]
Xenon fluorides are good fluorinating agents.[42]
Clathrates have been used for separation of He and Ne from Ar, Kr, and Xe, and also for the transportation of Ar, Kr, and Xe.[citation needed] (For instance, radioactive isotopes of krypton and xenon are difficult to store and dispose, and compounds of these elements may be more easily handled than the gaseous forms.[4]) In addition, clathrates of radioisotopes may provide suitable formulations for experiments requiring sources of particular types of radiation; hence. 85Kr clathrate provides a safe source of beta particles, while 133Xe clathrate provides a useful source of gamma rays.[43]
References
[edit]- ^ Smith GL, Mercier HP, Schrobilgen GJ (February 2007). "Synthesis of [F3S≡NXeF][AsF6] and structural study by multi-NMR and Raman spectroscopy, electronic structure calculations, and X-ray crystallography". Inorganic Chemistry. 46 (4): 1369–78. doi:10.1021/ic061899+. PMID 17256847.
- ^ Smith GL, Mercier HP, Schrobilgen GJ (May 2008). "F5SN(H)Xe+; a rare example of xenon bonded to sp3-hybridized nitrogen; synthesis and structural characterization of [F5SN(H)Xe][AsF6]". Inorganic Chemistry. 47 (10): 4173–84. doi:10.1021/ic702039f. PMID 18407626.
- ^ Pauling, Linus (June 1933). "The Formulas of Antimonic Acid and the Antimonates". J. Am. Chem. Soc. 55 (5): 1895–1900. doi:10.1021/ja01332a016.
- ^ a b c d e Holloway, John H. (1968). Noble-Gas Chemistry. London: Methuen. ISBN 0-416-03270-2.
- ^ Seppelt, Konrad (June 1979). "Recent developments in the Chemistry of Some Electronegative Elements". Accounts of Chemical Research. 12 (6): 211–216. doi:10.1021/ar50138a004.
- ^ Miessler, Gary L.; Tarr, Donald A. (1999). Inorganic Chemistry (2nd ed.). Prentice Hall. p. 272. ISBN 0-13-841891-8.
- ^ Bartlett, N. (1962). "Xenon hexafluoroplatinate Xe+[PtF6]−". Proceedings of the Chemical Society of London (6): 218. doi:10.1039/PS9620000197.
- ^ Graham, L.; Graudejus, O.; Jha N.K.; Bartlett, N. (2000). "Concerning the nature of XePtF6". Coordination Chemistry Reviews. 197: 321–334. doi:10.1016/S0010-8545(99)00190-3.
- ^ Claassen, H. H.; Selig, H.; Malm, J. G. (1962). "Xenon Tetrafluoride". J. Am. Chem. Soc. 84 (18): 3593. doi:10.1021/ja00877a042.
- ^ Hoppe, R.; Daehne, W.; Mattauch, H.; Roedder, K. (1962-11-01). "Fluorination of Xenon". Angew. Chem. Int. Ed. Engl. 1 (11): 599. doi:10.1002/anie.196205992.
- ^ a b Lehmann, J (2002). "The chemistry of krypton". Coordination Chemistry Reviews. 233–234: 1–39. doi:10.1016/S0010-8545(02)00202-3.
- ^ Brock, David S.; Mercier, Hélène P. A.; Schrobilgen, Gary J. (2013). "[H(OXeF2)n][AsF6] and [FXeII(OXeIVF2)n][AsF6] (n = 1, 2): Examples of Xenon(IV) Hydroxide Fluoride and Oxide Fluoride Cations and the Crystal Structures of [F3Xe—FH][Sb2F11] and [H5F4][SbF6]·2[F3Xe—FH][Sb2F11]". Journal of the American Chemical Society. 135 (13): 5089–5104. doi:10.1021/ja312493j. PMID 23398504.
- ^ Hutchinson, M. H. R. (1980). "Excimers and excimer lasers". Applied Physics. 21 (2): 95–114. Bibcode:1980ApPhy..21...95H. doi:10.1007/BF00900671. S2CID 93808742.
- ^ Drews, Thomas; Seppelt, Konrad (1997). "The Xe2+ Ion—Preparation and Structure". Angewandte Chemie International Edition. 36 (3): 273–274. doi:10.1002/anie.199702731.
- ^ Prusakov, V. N.; Sokolov, V. B. (1971). "Krypton difluoride". Soviet Atomic Energy. 31 (3): 990–999. doi:10.1007/BF01375764. S2CID 189775335.
- ^ John H. Holloway; Eric G. Hope (1998). A. G. Sykes (ed.). Advances in Inorganic Chemistry. Academic Press. p. 57. ISBN 0-12-023646-X.
- ^ Khriachtchev, L., Pettersson, M., Runeberg, N., Lundell, J., Räsänen, M. (2000). "A stable argon compound". Nature. 406 (6798): 874–876. Bibcode:2000Natur.406..874K. doi:10.1038/35022551. PMID 10972285. S2CID 4382128.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Bochenkova, Anastasia V.; Bochenkov, Vladimir E.; Khriachtchev, Leonid (2 July 2009). "HArF in Solid Argon Revisited: Transition from Unstable to Stable Configuration". The Journal of Physical Chemistry A. 113 (26): 7654–7659. Bibcode:2009JPCA..113.7654B. doi:10.1021/jp810457h. PMID 19243121.
- ^ Wyatt, J. R.; Strattan, L. W.; Snyder, S. C.; Hierl, P. M. (1975). "Chemical accelerator studies of reaction dynamics: Ar+
+ CH
4 → ArH+
+ CH
3" (PDF). The Journal of Chemical Physics. 62 (7): 2555. Bibcode:1975JChPh..62.2555W. doi:10.1063/1.430836. hdl:1808/16098. - ^ Barlow, M. J.; Swinyard, B. M.; Owen, P. J.; Cernicharo, J.; Gomez, H. L.; Ivison, R. J.; Krause, O.; Lim, T. L.; Matsuura, M.; Miller, S.; Olofsson, G.; Polehampton, E. T. (12 December 2013). "Detection of a Noble Gas Molecular Ion, 36
ArH+
, in the Crab Nebula". Science. 342 (6164): 1343–1345. arXiv:1312.4843. Bibcode:2013Sci...342.1343B. doi:10.1126/science.1243582. PMID 24337290. S2CID 37578581. - ^ Frenking, Gernot; Koch, Wolfram; Deakyne, Carol A.; Liebman, Joel F.; Bartlett, Neil (January 1989). "The ArF+ cation. Is it stable enough to be isolated in a salt?". Journal of the American Chemical Society. 111 (1): 31–33. doi:10.1021/ja00183a005.
- ^ Selig, Henry; Holloway, John H. (27 May 2005). Cationic and anionic complexes of the noble gases. Topics in Current Chemistry. Vol. 124. pp. 33–90. doi:10.1007/3-540-13534-0_2. ISBN 978-3-540-13534-0. S2CID 91636049.
- ^ "Periodic Table of Elements: Los Alamos National Laboratory". periodic.lanl.gov. Retrieved 2019-12-13.
- ^ Hogness, T.R.; Lunn, E.G. (1925). "The Ionization of Hydrogen by Electron Impact as Interpreted by Positive Ray Analysis". Phys. Rev. Lett. 26 (1). The American Physical Society: 44–55. Bibcode:1925PhRv...26...44H. doi:10.1103/PhysRev.26.44. Retrieved 15 December 2013.
- ^ Crew, Bec (7 February 2017). "Forget What You've Learned – Scientists Just Created a Stable Helium Compound". ScienceAlert. Retrieved 2019-12-13.
- ^ Kenneth S. Pitzer (1975). "Fluorides of radon and element 118" (PDF). J. Chem. Soc., Chem. Commun. (18): 760b–761. doi:10.1039/C3975000760b.
- ^ Moody, Ken (2013-11-30). "Synthesis of Superheavy Elements". In Schädel, Matthias; Shaughnessy, Dawn (eds.). The Chemistry of Superheavy Elements (2nd ed.). Springer Science & Business Media. pp. 24–8. ISBN 9783642374661.
- ^ Fricke, Burkhard (1975). Superheavy elements: a prediction of their chemical and physical properties. Structure and Bonding. Vol. 21. pp. 89–144. doi:10.1007/BFb0116498. ISBN 978-3-540-07109-9. Retrieved 4 October 2013.
{{cite book}}:|journal=ignored (help) - ^ a b c Kleppe, Annette K.; Amboage, Mónica; Jephcoat, Andrew P. (2014). "New high-pressure van der Waals compound Kr(H2)4 discovered in the krypton-hydrogen binary system". Scientific Reports. 4: 4989. Bibcode:2014NatSR...4.4989K. doi:10.1038/srep04989.
- ^ Lim, Sol Geo; Lee, Jong-Won; Fujihisa, Hiroshi; Oh, Chang Yeop; Jang, Jiyeong; Moon, Dohyun; Takeya, Satoshi; Muraoka, Michihiro; Yamamoto, Yoshitaka; Yoon, Ji-Ho (2023). "Neon encapsulation by a hydroquinone organic crystalline clathrate under ambient conditions". Communications Materials. 4 (1): 51. Bibcode:2023CoMat...4...51L. doi:10.1038/s43246-023-00378-z. S2CID 259583357.
- ^ Gunawardane, R. P.; Gies, H.; Liebau, F. (March 1987). "Studies on Clathrasils. X. The effect of "help gases" on the formation and stability of clathrasils". Zeitschrift für anorganische und allgemeine Chemie (in German). 546 (3): 189–198. doi:10.1002/zaac.19875460321.
- ^ Vos, W. L.; Finger, L. W.; Hemley, R. J.; Hu, J. Z.; Mao, H. K.; Schouten, J. A. (1992). "A high-pressure van der Waals compound in solid nitrogen-helium mixtures". Nature. 358 (6381): 46–48. Bibcode:1992Natur.358...46V. doi:10.1038/358046a0. S2CID 4313676.
- ^ Grochala, Wojciech (Oct 2007) [12 April 2007]. "Atypical compounds of gases, which have been called 'noble'". Chemical Society Reviews. 36 (10). Royal Society of Chemistry. p. 1638 and fn. 53–55. doi:10.1039/b702109g – via CiteSeerX.
- ^ Pauling, L. (1961). "A molecular theory of general anesthesia". Science. 134 (3471): 15–21. Bibcode:1961Sci...134...15P. doi:10.1126/science.134.3471.15. PMID 13733483. Reprinted as Pauling, Linus; Kamb, Barclay, eds. (2001). Linus Pauling: Selected Scientific Papers. Vol. 2. River Edge, New Jersey: World Scientific. pp. 1328–1334. ISBN 981-02-2940-2.
- ^ Henderson, W. (2000). Main group chemistry. Great Britain: Royal Society of Chemistry. p. 148. ISBN 0-85404-617-8.
- ^ Ikeda, Tomoko; Mae, Shinji; Yamamuro, Osamu; Matsuo, Takasuke; Ikeda, Susumu; Ibberson, Richard M. (November 23, 2000). "Distortion of Host Lattice in Clathrate Hydrate as a Function of Guest Molecule and Temperature". Journal of Physical Chemistry A. 104 (46): 10623–10630. Bibcode:2000JPCA..10410623I. doi:10.1021/jp001313j.
- ^ Saunders, M.; Jiménez-Vázquez, H. A.; Cross, R. J. & Poreda, R. J. (1993). "Stable compounds of helium and neon. He@C60 and Ne@C60". Science. 259 (5100): 1428–1430. Bibcode:1993Sci...259.1428S. doi:10.1126/science.259.5100.1428. PMID 17801275. S2CID 41794612.
- ^ Saunders, Martin; Jimenez-Vazquez, Hugo A.; Cross, R. James; Mroczkowski, Stanley; Gross, Michael L.; Giblin, Daryl E. & Poreda, Robert J. (1994). "Incorporation of helium, neon, argon, krypton, and xenon into fullerenes using high pressure". J. Am. Chem. Soc. 116 (5): 2193–2194. doi:10.1021/ja00084a089.
- ^ Mercier, H. P. A.; Moran, M. D.; Schrobilgen, G. J.; Steinberg, C.; Suontamo, R. J. (2004). "The Syntheses of Carbocations by Use of the Noble-Gas Oxidant, [XeOTeF
5][Sb(OTeF
5)
6]: The Syntheses and Characterization of the CX+
3 (X = Cl, Br, OTeF
5) and CBr(OTeF
5)+
2 Cations and Theoretical Studies of CX+
3 and BX
3 (X = F, Cl, Br, I, OTeF
5)". J. Am. Chem. Soc. 126 (17): 5533–5548. doi:10.1021/ja030649e. PMID 15113225. - ^ Christe, KO; Wilson, WW (Dec 1982). "Perfluoroammonium and alkali-metal salts of the heptafluoroxenate(VI) and octafluoroxenate(VI) anions". Inorganic Chemistry. 21 (12): 4113–4117. doi:10.1021/ic00142a001.
- ^ Christe, Karl O., Wilson, William W. Perfluoroammonium salt of heptafluoroxenon anion. U.S. patent 4,428,913, June 24, 1982
- ^ Christe, Karl O.; Dixon, David A. (1992). "A Quantitative Scale for the Oxidizing Strength of Oxidative Fluorinators". Journal of the American Chemical Society. 114 (8): 2978–2985. doi:10.1021/ja00034a033.
- ^ Bhatnagar, Vijay M. (1963). "Clathrate compounds of quinol". Defence Science Journal, Supplement. 13 (4): 57–66.
Resources
[edit]- Khriachtchev, Leonid; Räsänen, Markku; Gerber, R. Benny (2009). "Noble-Gas Hydrides: New Chemistry at Low Temperatures". Accounts of Chemical Research. 42 (1): 183–91. doi:10.1021/ar800110q. PMID 18720951.
