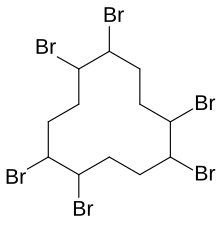
Hexabromocyclododecane
| |
| Names | |
|---|---|
| Preferred IUPAC name
1,2,5,6,9,10-Hexabromocyclododecane | |
| Other names
Hexabromocyclododecane
| |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | HBCDD HBCD |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.019.724 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H18Br6 | |
| Molar mass | 641.7 g/mol |
| Melting point | 186 °C (367 °F; 459 K) (175–195 °C, depending upon isomer) |
| 3.4 µg/L in water | |
| Hazards | |
| GHS labelling: | |
  
| |
| Warning | |
| H315, H319, H335, H361, H362, H410 | |
| P201, P202, P260, P261, P263, P264, P270, P271, P273, P280, P281, P302+P352, P304+P340, P305+P351+P338, P308+P313, P312, P321, P332+P313, P337+P313, P362, P391, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
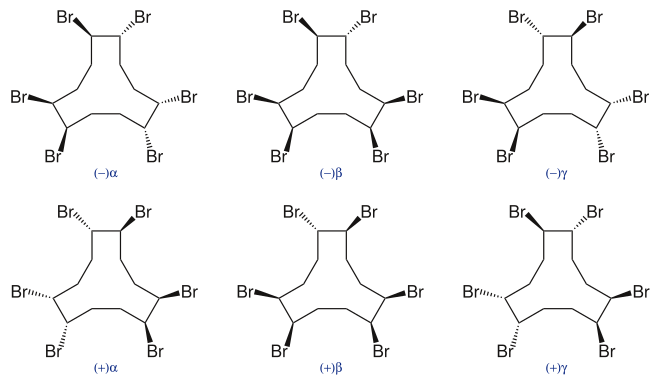
Hexabromocyclododecane (HBCD or HBCDD) is a brominated flame retardant. It consists of twelve carbon, eighteen hydrogen, and six bromine atoms tied to the ring. Its primary application is in extruded (XPS) and expanded (EPS) polystyrene foam used as thermal insulation in construction. Other uses are upholstered furniture, automobile interior textiles, car cushions and insulation blocks in trucks, packaging material, video cassette recorder housing, and electric and electronic equipment. According to UNEP, "HBCD is produced in China, Europe, Japan, and the USA. The last known current annual production is approximately 28,000 tonnes per year. The main share of the market volume is used in Europe and China" (figures from 2009 to 2010).[2] Due to its persistence, toxicity, and ecotoxicity, the Stockholm Convention on Persistent Organic Pollutants decided in May 2013 to list hexabromocyclododecane in Annex A to the convention with specific exemptions for production and use in expanded polystyrene and extruded polystyrene in buildings. Because HBCD has 16 possible stereo-isomers with different biological activities, the substance poses a difficult problem for manufacture and regulation.
Toxicity
[edit]HBCD's toxicity and its harm to the environment are current sources of concern. HBCD can be found in environmental samples such as birds, mammals, fish, and other aquatic organisms as well as soil and sediment.[3] On this basis, on 28 October 2008, the European Chemicals Agency decided to include HBCD in the SVHC list,[4] Substances of Very High Concern, within the Registration, Evaluation, Authorisation and Restriction of Chemicals framework. On 18 February 2011, HBCD was listed in Annex XIV of REACH and hence is subject to Authorisation. HBCD can be used until the so-called “sunset date” (21 August 2015). After that date, only authorized applications will be allowed in the EU.
HBCD has been found widely present in biological samples from remote areas and supporting pieces of evidence for its classification as Persistent, Bioaccumulative, and Toxic (PBT) and undergoes long-range environmental transportation.[5] In July 2012, an EU-harmonized classification and labeling for HBCD entered into force. HBCD has been classified as a category 2 for reproductive toxicity.[6] Since August 2010 hexabromocyclododecanes are included in the EPA's List of Chemicals of Concern.[7] In May 2013 the Stockholm Convention on Persistent Organic Pollutants (POPs) decided to include HBCD in the convention's Annex A for elimination, with specific exemptions for expanded and extruded polystyrene in buildings needed to give countries time to phase-in safer substitutes. HBCD is listed for elimination, but with a specific exemption for expanded polystyrene (EPS) and extruded polystyrene (XPS) in buildings. Countries may choose to use this exemption for up to five years after the request for exemption is submitted.[8] Japan was the first country to implement a ban on the import and production of HBCD effective in May 2014.
Because HBCD has 16 possible stereo-isomers with different biological activities, the substance poses a difficult problem for manufacture and regulation.[9] The HBCD commercial mixture is composed of three main diastereomers denoted as alpha (α-HBCD), beta (β-HBCD), and gamma (γ-HBCD) with traces of others. A series of four published in vivo mice studies were conducted between several federal and academic institutions to characterize the toxicokinetic profiles of individual HBCD stereoisomers. The predominant diastereomer in the HBCD mixture, γ-HBCD, undergoes rapid hepatic metabolism, fecal and urinary elimination, and biological conversion to other diastereomers with a short biological half-life of 1–4 days. After oral exposure to the γ-HBCD diastereomer, β-HBCD was detected in the liver and brain, and α-HBCD and β-HBCD was detected in the fat and feces [10] with multiple novel metabolites identified - monohydroxy-pentabromocyclododecane, monohydroxy-pentabromocyclododecene, dihydroxy-pentabromocyclododecene, and dihydroxy-pentabromocyclododecadiene.[11] In contrast, α-HBCD is more biologically persistent, resistant to metabolism, bioaccumulates in lipid-rich tissues after a 10-day repeated exposure study, and has a longer biological half-life of up to 21 days; only α-HBCD was detected in the liver, brain, fat and feces with no stereoisomerization to γ-HBCD or β-HBCD and low trace levels of four different hydroxylated metabolites were identified.[12] Developing mice had higher HBCD tissue levels than adult mice after exposure to either α-HBCD or γ-HBCD indicating the potential for increased susceptibility of the developing young to HBCD effects.[13] The reported toxicokinetic differences of individual HBCD diastereoisomers have important implications for the extrapolation of toxicological studies of the commercial HBCD mixture to the assessment of human risk.
Environmental Concerns
[edit]This section needs to be updated. (January 2020) |
Due to its persistence, toxicity, and ecotoxicity, the Stockholm Convention on Persistent Organic Pollutants decided in May 2013 to list hexabromocyclododecane in Annex A to the convention with specific exemptions for production and use in expanded polystyrene and extruded polystyrene in buildings. Countries may choose to use this exemption for up to five years after the request for exemption is submitted.[14]
There is a large and still increasing stock of HBCD in the anthroposphere, mainly in EPS and XPS insulation boards.[15] A long-term environmental monitoring program run by the Fraunhofer Institute for Molecular Biology and Applied Ecology demonstrates a general trend that HBCD concentrations are decreasing over time.[16] HBCD emissions into the environment are controlled under the voluntary industry emission management program: the Voluntary Emissions Control Action Programme (VECAP).[17] The VECAP annual report demonstrates a continuous decrease of potential emissions of HBCD to the environment.[18]
References
[edit]- ^ https://treaties.un.org/doc/Publication/CN/2013/CN.934.2013-Eng.pdf [bare URL PDF]
- ^ ^ UNEP Stockholm Convention HBCD Risk management evaluation http://chm.pops.int/Convention/POPsReviewCommittee/Chemicals/tabid/243/Default.aspx
- ^ Covaci, A; Gerecke, AC; Law, RJ; Voorspoels, S; Kohler, M; Heeb, NV; Leslie, H; Allchin, CR; De Boer, J (2006). "Hexabromocyclododecanes (HBCDs) in the environment and humans: A review" (PDF). Environmental Science & Technology. 40 (12): 3679–88. Bibcode:2006EnST...40.3679C. doi:10.1021/es0602492. PMID 16830527.
- ^ "ECHA SVHC Official List". Echa.europa.eu. 2011-12-19. Archived from the original on 2009-03-18. Retrieved 2012-06-20.
- ^ "ECHA HBCD SVHC Supporting Documentation" (PDF). Echa.europa.eu. Archived from the original (PDF) on 2011-10-26. Retrieved 2012-06-20.
- ^ Commission Regulation (EU) No 618/2012 of 10 July 2012 amending, for the purposes of its adaptation to technical and scientific progress, Regulation (EC) No 1272/2008 of the European Parliament and of the Council on classification, labelling and packaging of substances and mixtures
- ^ "EPA action details on HBCD". Epa.gov. Archived from the original on 2012-05-11. Retrieved 2012-06-20.
- ^ https://treaties.un.org/doc/Publication/CN/2013/CN.934.2013-Eng.pdf [bare URL PDF]
- ^ "Hexabromocyclododecane Challenges Scientists and Regulators". Retrieved 2012-06-20.
- ^ Szabo DT, Diliberto JJ, Hakk H, Huwe JK, Birnbaum LS (2010). "Toxicokinetics of the flame retardant hexabromocyclododecane gamma: effect of dose, timing, route, repeated exposure, and metabolism". Toxicological Sciences. 117 (2): 282–93. doi:10.1093/toxsci/kfq183. PMID 20562218.
- ^ Hakk H, Szabo DT, Huwe J, Diliberto J, Birnbaum LS (2012). "Novel and distinct metabolites identified following a single oral dose of α- or γ-hexabromocyclododecane in mice". Environmental Science and Technology. 46 (24): 13494–503. Bibcode:2012EnST...4613494H. doi:10.1021/es303209g. PMC 3608416. PMID 23171393.
- ^ Szabo DT, Diliberto JJ, Hakk H, Huwe JK, Birnbaum LS (2011). "Toxicokinetics of the flame retardant hexabromocyclododecane alpha: effect of dose, timing, route, repeated exposure, and metabolism". Toxicological Sciences. 121 (2): 234–44. doi:10.1093/toxsci/kfr059. PMID 21441408.
- ^ Szabo DT, Diliberto JJ, Huwe JK, Birnbaum LS (2011). "Differences in tissue distribution of HBCD alpha and gamma between adult and developing mice". Toxicological Sciences. 123 (1): 256–63. doi:10.1093/toxsci/kfr161. PMID 21705717.
- ^ https://treaties.un.org/doc/Publication/CN/2013/CN.934.2013-Eng.pdf [bare URL PDF]
- ^ Dynamic Substance Flow Analysis Model for Selected Brominated Flame Retardants as a Base for Decision Making on Risk Reduction Measures, study for the Swiss National Science Foundation, 2007
- ^ Fraunhofer: H. Rüdel, J. Müller, M. Quack, R. Klein, 2012: Monitoring of hexabromo¬cyclodo¬decane diastereomers in fish from European freshwaters and estuaries. Environ. Sci. Pollut. Res. 19, 772-783 “Environmental Monitoring of HBCD in Europe” Society for Environmental Toxicology and Chemistry Europe – SETAC: Ecosystem Protection in a Sustainable World: a challenge for science and regulation. 2011. http://publica.fraunhofer.de/documents/N-217320.html
- ^ VECAP website: www.vecap.info
- ^ "Maintaining Momentum European Annual Progress Report 2012".
External links
[edit]- MPI Milebrome B-972, FR 50 & GC SAM: The low-cost alternatives to Hexabromocyclododecane (HBCD) in EPS and XPS applications Archived 2013-11-12 at the Wayback Machine, Stockholm Convention on Persistent Organic Pollutants 2012
- An Overview of Alternatives to Tetrabromobisphenol A (TBBPA) and Hexabromocyclododecane (HBCD) Archived 2010-02-15 at the Wayback Machine, University of Massachusetts Lowell, March 2006
- ECHA: MEMBER STATE COMMITTEE SUPPORT DOCUMENT FOR IDENTIFICATION OF HEXABROMOCYCLODODECANE AND ALL MAJOR DIASTEREOISOMERS IDENTIFIED AS A SUBSTANCE OF VERY HIGH CONCERN, 8 October 2008
- Factsheet BSEF
- BSEF – the bromine industry website’s page on HBCD Archived 2006-08-29 at the Wayback Machine

