Dydrogesterone
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Duphaston, others[1] |
| Other names | Isopregnenone; Dehydroprogesterone; Didrogesteron; 6-Dehydroretroprogesterone; 9β,10α-Pregna-4,6-diene-3,20-dione; NSC-92336[2][3] |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth |
| Drug class | Progestogen; Progestin |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 28%[4][5] |
| Protein binding | ? (probably to albumin)[6][7] |
| Metabolism | Hepatic: AKR1C1, AKR1C3, CYP3A4[10][8] |
| Metabolites | 20α-DHD (exclusively via AKR1C1 and AKRC13)[8] |
| Elimination half-life | Parent: 5–7 hours[9] Metabolite: 14–17 hours[9] |
| Excretion | Urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.005.280 |
| Chemical and physical data | |
| Formula | C21H28O2 |
| Molar mass | 312.453 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 144 °C (291 °F) |
| Boiling point | 463 °C (865 °F) |
| Solubility in water | Insoluble mg/mL (20 °C) |
| |
| |
| (verify) | |
Dydrogesterone, sold under the brand name Duphaston among others,[1] is a progestin medication which is used for a variety of indications, including threatened or recurrent miscarriage during pregnancy, dysfunctional bleeding, infertility due to luteal insufficiency, dysmenorrhea, endometriosis, secondary amenorrhea, irregular cycles, premenstrual syndrome, and as a component of menopausal hormone therapy.[7] It is taken by mouth.[7]
Side effects of dydrogesterone include menstrual irregularities, headache, nausea, breast tenderness, and others.[11][12] Dydrogesterone is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[7][13] The medication is an atypical progestogen and does not inhibit ovulation.[7][14] It has weak antimineralocorticoid activity and no other important hormonal activity.[7][13]
Dydrogesterone was developed in the 1950s and introduced for medical use in 1961.[15] It is available widely throughout Europe, including in the United Kingdom, and is also marketed in Australia and elsewhere in the world.[3][15] The medication was previously available in the United States,[15] but it has been discontinued in that country.[16]
Medical uses
[edit]Dydrogesterone has proven effective in a variety of conditions associated with progesterone deficiency,[17] Infertility due to luteal insufficiency[18][19] including threatened miscarriage,[20] habitual or recurrent miscarriage,[21] Menstrual disorders[22] premenstrual syndrome,[23] and endometriosis.[24] Dydrogesterone has also been registered as a component of menopausal hormone therapy[25] to counteract the effects of unopposed estrogen on the endometrium in persons with an intact uterus.
Gynecological disorders
[edit]Primary or essential dysmenorrhea is a very common gynecological phenomenon experienced by women during their reproductive years. Clinical studies have shown symptom relief and a reduction in pain with dydrogesterone treatment for dysmenorrhea.[26] Secondary amenorrhea is not a specific disease, but is instead a symptom. Dydrogesterone has been found to adequately induce bleeding within a sufficiently estrogen-primed endometrium. When estradiol levels are found to be low, dydrogesterone treatment is more effective when supplemented with estrogens.[27]
Endometriosis is a chronic disease which can cause severe, progressive, and at times, incapacitating dysmenorrhea, pelvic pain, dyspareunia and infertility. Dydrogesterone relieves pain without inhibiting ovulation, so that patients are able to become pregnant during treatment. Dydrogesterone is particularly suitable in cases where the woman desires to become pregnant and to prevent bleeding problems.[28] Dydrogesterone results in statistically significant reductions in the symptoms pelvic pain, dysmenorrhea and dyspareunia after the first treatment cycle for the treatment of post-laparoscopic endometriosis.[26] The amount and duration of menstrual bleeding is also significantly reduced, and from the end of the third month onwards, bleeding was considered normal in the majority of patients. Improvement of endometriosis was observed in 71% of patients.
Dydrogesterone has shown reasonable efficacy in relieving a number of premenstrual syndrome symptoms like mood swings and physical symptoms.[23] Cyclic treatment with low-dose (10 mg/day) dydrogesterone has been found to be effective in the treatment of fibrocystic breast changes and associated breast pain.[29]
Infertility and miscarriage
[edit]Oral dydrogesterone is an effective medication, well tolerated and accepted among patients, and can be considered for routine luteal support. Advantage of dydrogesterone is oral administration, easy to use and better patient compliance which results in high satisfaction score of oral dydrogesterone in luteal support of IVF/ICSI cycles.[30] Oral administration of progestins dydrogesterone at least similar live birth rate than vaginal progesterone capsules when used for luteal support in embryo transfer, with no evidence of increased risk of miscarriage.[31][32]
Threatened miscarriage is defined as bleeding during the first 20 weeks of pregnancy while the cervix is closed. It is the most common complication in pregnancy, occurring in 20% of all pregnancies. Recurrent abortion is defined as the loss of three or more consecutive pregnancies. Dydrogesterone is associated with approximately two-fold significant reduction in the miscarriage rate as compared to standard care in threatened and recurrent miscarriages with minimal side effects.[21][33]
Hormone therapy
[edit]The objective behind menopausal hormone therapy is to actively increase the circulating levels of estrogen to control hot flashes and to prevent the long-term effects of the menopause, such as bone resorption and unfavourable changes in blood lipids. The administration of estradiol halts, or reverses atrophic changes that occur due to the loss of endogenous estradiol during the menopause.[34]
Estrogen promotes endometrial cell growth and in postmenopausal women with an intact uterus, estrogen monotherapy results in continued endometrial development without the physiological secretory changes normally brought on by progesterone. This action is associated with an increased incidence of endometrial hyperplasia and carcinoma. Additional protection with progestogens is therefore important in patients with an intact uterus who receive estrogen therapy. Dydrogesterone counters the proliferative effect of estrogens on the endometrium and ensures the transition to a secretory pattern and cyclical shedding of the endometrium in serial menopausal hormone therapy regimes. Dydrogesterone effectively protects against the ontogenesis of endometrial hyperplasia. Unlike androgenic progestogens, dydrogesterone does not reverse the benefits brought on by estradiol on lipid profiles and carbohydrate metabolism. In a continuous, combined menopausal hormone therapy regimen, dydrogesterone retards the proliferation of the endometrium so that it remains atrophic or inactive.[35]
Available forms
[edit]Dydrogesterone is available in the form of 10 mg oral tablets both alone and in combination with estradiol.[36][37]
Contraindications
[edit]Side effects
[edit]The most commonly reported medication-related adverse reactions in people taking dydrogesterone without an estrogen in clinical trials of indications have included menstrual irregularities, headaches, migraines, nausea, breast tenderness, bloating, and weight gain.[11][12] The use of progestins, in particular medroxyprogesterone acetate, in treating postmenopausal symptoms have been associated with increased risk of blood clots[38] and breast cancer in a study carried out by the Women's Health Initiative. While the study did not involve dydrogesterone, it is possible, but not certain, that it too increases these risks.[39]
Dydrogesterone has been prescribed and used in over 10 million pregnancies worldwide. There have been no harmful effects exhibited due to the use of dydrogesterone while pregnant. Dydrogesterone is safe to use during pregnancy only when prescribed and indicated by a medical practitioner.[40] Studies have not shown any incidence of decreased fertility due to dydrogesterone at therapeutic dose.[40] The Ames test found no evidence of any potential mutagenic or toxicity properties.[41]
| Study | Therapy | Hazard ratio (95% CI) |
|---|---|---|
| E3N-EPIC: Fournier et al. (2005) | Estrogen alone | 1.1 (0.8–1.6) |
| Estrogen plus progesterone Transdermal estrogen Oral estrogen |
0.9 (0.7–1.2) 0.9 (0.7–1.2) No events | |
| Estrogen plus progestin Transdermal estrogen Oral estrogen |
1.4 (1.2–1.7) 1.4 (1.2–1.7) 1.5 (1.1–1.9) | |
| E3N-EPIC: Fournier et al. (2008) | Oral estrogen alone | 1.32 (0.76–2.29) |
| Oral estrogen plus progestogen Progesterone Dydrogesterone Medrogestone Chlormadinone acetate Cyproterone acetate Promegestone Nomegestrol acetate Norethisterone acetate Medroxyprogesterone acetate |
Not analyzeda 0.77 (0.36–1.62) 2.74 (1.42–5.29) 2.02 (1.00–4.06) 2.57 (1.81–3.65) 1.62 (0.94–2.82) 1.10 (0.55–2.21) 2.11 (1.56–2.86) 1.48 (1.02–2.16) | |
| Transdermal estrogen alone | 1.28 (0.98–1.69) | |
| Transdermal estrogen plus progestogen Progesterone Dydrogesterone Medrogestone Chlormadinone acetate Cyproterone acetate Promegestone Nomegestrol acetate Norethisterone acetate Medroxyprogesterone acetate |
1.08 (0.89–1.31) 1.18 (0.95–1.48) 2.03 (1.39–2.97) 1.48 (1.05–2.09) Not analyzeda 1.52 (1.19–1.96) 1.60 (1.28–2.01) Not analyzeda Not analyzeda | |
| E3N-EPIC: Fournier et al. (2014) | Estrogen alone | 1.17 (0.99–1.38) |
| Estrogen plus progesterone or dydrogesterone | 1.22 (1.11–1.35) | |
| Estrogen plus progestin | 1.87 (1.71–2.04) | |
| CECILE: Cordina-Duverger et al. (2013) | Estrogen alone | 1.19 (0.69–2.04) |
| Estrogen plus progestogen Progesterone Progestins Progesterone derivatives Testosterone derivatives |
1.33 (0.92–1.92) 0.80 (0.44–1.43) 1.72 (1.11–2.65) 1.57 (0.99–2.49) 3.35 (1.07–10.4) | |
| Footnotes: a = Not analyzed, fewer than 5 cases. Sources: See template. | ||
| Study | Therapy | Hazard ratio (95% CI) |
|---|---|---|
| E3N-EPIC: Fournier et al. (2005)a | Transdermal estrogen plus progesterone <2 years 2–4 years ≥4 years |
0.9 (0.6–1.4) 0.7 (0.4–1.2) 1.2 (0.7–2.0) |
| Transdermal estrogen plus progestin <2 years 2–4 years ≥4 years |
1.6 (1.3–2.0) 1.4 (1.0–1.8) 1.2 (0.8–1.7) | |
| Oral estrogen plus progestin <2 years 2–4 years ≥4 years |
1.2 (0.9–1.8) 1.6 (1.1–2.3) 1.9 (1.2–3.2) | |
| E3N-EPIC: Fournier et al. (2008) | Estrogen plus progesterone <2 years 2–4 years 4–6 years ≥6 years |
0.71 (0.44–1.14) 0.95 (0.67–1.36) 1.26 (0.87–1.82) 1.22 (0.89–1.67) |
| Estrogen plus dydrogesterone <2 years 2–4 years 4–6 years ≥6 years |
0.84 (0.51–1.38) 1.16 (0.79–1.71) 1.28 (0.83–1.99) 1.32 (0.93–1.86) | |
| Estrogen plus other progestogens <2 years 2–4 years 4–6 years ≥6 years |
1.36 (1.07–1.72) 1.59 (1.30–1.94) 1.79 (1.44–2.23) 1.95 (1.62–2.35) | |
| E3N-EPIC: Fournier et al. (2014) | Estrogens plus progesterone or dydrogesterone <5 years ≥5 years |
1.13 (0.99–1.29) 1.31 (1.15–1.48) |
| Estrogen plus other progestogens <5 years ≥5 years |
1.70 (1.50–1.91) 2.02 (1.81–2.26) | |
| Footnotes: a = Oral estrogen plus progesterone was not analyzed because there was a low number of women who used this therapy. Sources: See template. | ||
Overdose
[edit]There is not enough clinical data to support overdose in humans. The maximum dose of dydrogesterone administered to humans to date was 360 mg orally, and the medication was found to be well tolerated at this dose.[citation needed] There are no antidotes to overdose, and treatment should be based on symptoms.[40] In acute toxicity trials, the LD50 doses in rats were in excess of 4,640 mg/kg orally.[42][43]
Interactions
[edit]In menopausal hormone therapy, dydrogesterone is administered together with an estrogen. Therefore, the interaction between dydrogesterone and estrogens has been assessed, and no clinically significant interaction has been observed.[citation needed]
Pharmacology
[edit]Pharmacodynamics
[edit]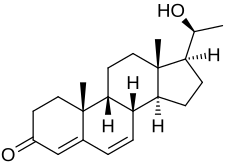
Dydrogesterone is a highly selective progestogen, and due to its unique structure, unlike progesterone and many other progestins, binds almost exclusively to the progesterone receptor (PR).[44] The affinity of dydrogesterone for the PR is relatively low at about 16% of that of progesterone.[45][46] However, in vivo, dydrogesterone is comparatively much more potent by the oral route, with an equivalent dose, in terms of endometrial proliferation, that is 10 to 20 times lower than that of progesterone.[47] This is due to pharmacokinetic differences between the two medications, namely improved bioavailability and metabolic stability with dydrogesterone as well as additional progestogenic activity of its metabolites.[13] Dydrogesterone binds to and activates both of the major isoforms of the PR, the PR-A and PR-B, with a similar selectivity ratio between the two receptors as that of progesterone and with lower efficacy at the receptors relative to progesterone.[45] The major active metabolite of dydrogesterone, 20α-dihydrodydrogesterone (20α-DHD), has progestogenic activity as well but with greatly decreased potency relative to dydrogesterone.[45] As with other progestogens, dydrogesterone has functional antiestrogenic effects in certain tissues, for instance in the endometrium, and induces endometrial secretory transformation.[7]
Dydrogesterone does not bind importantly to the androgen, estrogen, or glucocorticoid receptor.[46][45] As such, it is devoid of androgenic or antiandrogenic, estrogenic or antiestrogenic, and glucocorticoid or antiglucocorticoid activity.[44][7][45] Similarly to progesterone however, dydrogesterone binds to the mineralocorticoid receptor and possesses antimineralocorticoid activity, but only weakly so.[7][45] Like other progestins but unlike progesterone, which forms sedative neurosteroid metabolites, dydrogesterone is not able to be metabolized in a similar way, and for this reason, is non-sedative.[7] The medication and 20α-DHD do not inhibit 5α-reductase.[45] Dydrogesterone has been found to inhibit myometrium contractility via an undefined progesterone receptor-independent mechanism in vivo in pregnant rats and in vitro in human tissue at concentrations at which progesterone and other progestogens do not.[48]
Atypical progestogenic profile
[edit]Due to its progestogenic activity, dydrogesterone can produce antigonadotropic effects at sufficient doses in animals.[49] However, it does not suppress secretion of the gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), or inhibit ovulation at typical clinical dosages in humans.[7][14][50] Oral doses of dydrogesterone of 5 to 40 mg/day on days 5 to 25 of the cycle fail to suppress ovulation (assessed by urinary pregnanediol and laparotomy), and one study found that ovulation persisted even in women treated with an oral dosage of as great as 400 mg/day (assessed by visual inspection of the ovaries).[51][14] Likewise, an intramuscular injection of 100 mg dydrogesterone in microcrystalline aqueous suspension on the first to third day of the cycle did not interfere with the development of an ovulatory pattern of spontaneous uterine contractions in women.[14][52] A couple of conflicting studies exist on the issue of ovulation inhibition by dydrogesterone however, with findings of partial or full inhibition of ovulation by oral dydrogesterone.[14] This included prevention of the mid-cycle LH and FSH peaks and the luteal-phase rise in body temperature and pregnanediol excretion.[14] Nonetheless, the overall consensus among researchers seems to be that dydrogesterone does not inhibit ovulation in women.[14] The apparent inability of dydrogesterone to prevent ovulation is in contrast to all other clinically used progestogens except trengestone, which is closely related to dydrogesterone.[51][53] Similarly to trengestone but also unlike all other clinically used progestogens, dydrogesterone does not have a hyperthermic effect in humans (i.e., it does not increase body temperature).[7][53][54]
It has been said that the lack of ovulation inhibition and hyperthermic effect with retroprogesterone derivatives like dydrogesterone may represent a dissociation of peripheral and central progestogenic activity.[55][56] However, a related retroprogesterone derivative, trengestone, likewise does not inhibit ovulation or produce a hyperthermic effect but rather has an inducing effect on ovulation.[53]
Whereas all other assessed progestins are associated with an increased risk of breast cancer when combined with an estrogen in postmenopausal women, neither oral progesterone nor dydrogesterone are associated with a significantly increased risk of breast cancer (although the risk of breast cancer is non-significantly higher with dydrogesterone).[57][58][59] Similarly, like oral progesterone but in contrast to other progestins, dydrogesterone does not appear to further increase the risk of venous thromboembolism when used in combination with an oral estrogen.[60][61] Dydrogesterone may also provide inferior endometrial protection relative to other progestins such as medroxyprogesterone acetate and norethisterone acetate, with a significantly increased risk of endometrial cancer in combination with an estrogen with long-term therapy (>5 years).[62][63][64]
Other activity
[edit]Dydrogesterone weakly stimulates the proliferation of MCF-7 breast cancer cells in vitro, an action that is independent of the classical PRs and is instead mediated via the progesterone receptor membrane component-1 (PGRMC1).[65] Certain other progestins are also active in this assay, whereas progesterone acts neutrally.[65] It is unclear if these findings may explain the different risks of breast cancer observed with progesterone, dydrogesterone, and other progestins such as medroxyprogesterone acetate and norethisterone in clinical studies.[66]
Pharmacokinetics
[edit]Absorption
[edit]Dydrogesterone and its major metabolite, 20α-DHD, have predictable pharmacokinetics. The single-dose kinetics are linear in the oral dose range of 2.5 to 10 mg. The pharmacokinetics do not change during repeated administration of up to 20 mg dydrogesterone once daily. Dydrogesterone is readily absorbed with oral administration. The absolute bioavailability of dydrogesterone is on average 28%.[4] Tmax values vary between 0.5 and 2.5 hours.[67] Steady state is attained after 3 days of treatment.[40] The levels of 20α-DHD, which is the main active metabolite, are also found to peak about 1.5 hours post-dose.[40]
A single intramuscular injection of 100 mg dydrogesterone in microcrystalline aqueous suspension has been found to have a duration of action of 16 to 38 days in terms of clinical biological effect in the uterus in women.[14] This was specifically the time until the onset of withdrawal bleeding in estrogen-treated amenorrheic women.[14]
| Compound | Form | Dose for specific uses (mg)[c] | DOA[d] | |||
|---|---|---|---|---|---|---|
| TFD[e] | POICD[f] | CICD[g] | ||||
| Algestone acetophenide | Oil soln. | – | – | 75–150 | 14–32 d | |
| Gestonorone caproate | Oil soln. | 25–50 | – | – | 8–13 d | |
| Hydroxyprogest. acetate[h] | Aq. susp. | 350 | – | – | 9–16 d | |
| Hydroxyprogest. caproate | Oil soln. | 250–500[i] | – | 250–500 | 5–21 d | |
| Medroxyprog. acetate | Aq. susp. | 50–100 | 150 | 25 | 14–50+ d | |
| Megestrol acetate | Aq. susp. | – | – | 25 | >14 d | |
| Norethisterone enanthate | Oil soln. | 100–200 | 200 | 50 | 11–52 d | |
| Progesterone | Oil soln. | 200[i] | – | – | 2–6 d | |
| Aq. soln. | ? | – | – | 1–2 d | ||
| Aq. susp. | 50–200 | – | – | 7–14 d | ||
|
Notes and sources:
| ||||||
Distribution
[edit]The plasma protein binding of dydrogesterone and 20α-DHD are unknown. Based on the plasma protein binding of other progestins however, they are probably bound to albumin and not to sex hormone-binding globulin or corticosteroid-binding globulin.[6][7]
Metabolism
[edit]The metabolism of dydrogesterone occurs in the liver.[87] It is virtually completely metabolized.[87] The primary metabolic pathway is the hydrogenation of the 20-keto group mainly by AKR1C1 and to a lesser extent AKR1C3, resulting in 20α-DHD. This active metabolite is a progestogen similarly to dydrogesterone, albeit with much lower potency.[8] With oral administration of dydrogesterone, circulating levels of 20α-DHD are substantially higher than those of dydrogesterone.[45] The ratios of 20α-DHD to dydrogesterone in terms of peak levels and area-under-the-curve (AUC) levels have been found to be 25:1 and 40:1, respectively.[45] For these reasons, despite the lower relative progestogenic potency of 20α-DHD, dydrogesterone may act as a prodrug of this metabolite.[45]
The metabolism of dydrogesterone differs from progesterone.[14] Whereas the major metabolite of progesterone is pregnanediol, the corresponding derivative of dydrogesterone, retropregnanediol, cannot be detected in urine with oral administration of dydrogesterone.[14] All of the metabolites of dydrogesterone retain the 4,6-diene-3-one structure and are metabolically stable. As such, similarly to progesterone, dydrogesterone does not undergo aromatization.
The mean elimination half-lives of dydrogesterone and 20α-DHD are in the ranges of 5 to 7 hours and 14 to 17 hours, respectively.[9]
Excretion
[edit]Dydrogesterone and its metabolites are excreted predominantly in urine. Total clearance of plasma is at a rate of 6.4 L/min. Within 72 hours, excretion is virtually complete. 20α-DHD is preponderantly present in the urine as a conjugate of glucuronic acid. Approximately 85% of the oral dose is successfully removed from the body within 24 hours. Around 90% of excreted material is 20α-DHD.[14]
Miscellaneous
[edit]The pharmacokinetics of dydrogesterone have been reviewed.[7][88]
Chemistry
[edit]
Dydrogesterone, also known as 6-dehydro-9β,10α-progesterone or as 9β,10α-pregna-4,6-diene-3,20-dione, is a synthetic pregnane steroid and a derivative of progesterone and retroprogesterone (9β,10α-progesterone).[2][3] Retroprogesterone derivatives like dydrogesterone are analogues of progesterone in which the hydrogen atom at the 9th carbon has been switched from the α-position (below the plane) to the β-position (above the plane) and the methyl group at the 10th carbon has been switched from the β-position to the α-position.[53] This reversed configuration in dydrogesterone results in a "bent" spatial geometry in which the plane of rings A and B is orientated at a 60° angle below the rings C and D.[7] Dydrogesterone also has an additional double bond between the C6 and C7 positions (4,6-dien-3-one configuration).[2][3] While its chemical structure is close to that of progesterone, these changes result in dydrogesterone having improved oral activity and metabolic stability, among other differences, in comparison to progesterone.[7][44]
Analogues
[edit]Other retroprogesterone derivatives, and analogues of dydrogesterone, include trengestone (1,6-didehydro-6-chlororetroprogesterone) and Ro 6-3129 (16α-ethylthio-6-dehydroretroprogesterone).[2][3]
Synthesis
[edit]Dydrogesterone is synthesized and manufactured by treatment of progesterone with ultraviolet light exposure.[44]
Chemical syntheses of dydrogesterone have been published.[88]
History
[edit]Dydrogesterone is a progestin which was first synthesized by Duphar in the 1950s and was first introduced to the market in 1961. It is unique, being the only retrosteroid that is commercially available and its molecular structure is closely related to that of natural progesterone,[13] but it has enhanced oral bioavailability. It is estimated that during the period from 1977 to 2005[89] around 38 million women were treated with dydrogesterone and that fetuses were exposed to dydrogesterone in utero in more than 10 million pregnancies. It has been approved in more than 100 countries worldwide. It is commercially marketed under the brand name Dydroboon and manufactured by Mankind Pharma. Dydrogesterone was first introduced, by Duphar, as Duphaston in the United Kingdom in 1961.[15] Subsequently, it was introduced in the United States as Duphaston and Gynorest in 1962 and 1968, respectively.[15] Duphaston was removed from the United States market in 1979,[90] and Gynorest is also no longer available in the United States.[91]
Society and culture
[edit]Generic names
[edit]Dydrogesterone is the generic name of the drug and its INN, USAN, and BAN, while dydrogestérone is its DCF and didrogesterone is its DCIT.[2][3][15][92] It was also originally known as isopregnenone.[2][3][15][92] Dydrogesterone has also been referred to as retroprogesterone, but should not be confused with retroprogesterone.[93]
Brand names
[edit]Dydrogesterone is marketed mainly under the brand names Duphaston (alone) and Femoston (in combination with estradiol).[92][3] It also is or has been marketed alone under the brand names Dabroston, Divatrone, Dufaston, Duvaron, Dydrofem, Femoston, Gestatron, Gynorest, Prodel, Retrone, Terolut and Zuviston and in combination with estradiol under the brand names Climaston, Femaston, and Femphascyl.[1][3][2][15]
Availability
[edit]Dydrogesterone is available widely throughout the world.[92][3] It is marketed in the United Kingdom, India, Ireland, South Africa, and Australia, but not in the United States, Canada, or New Zealand.[92][3] The medication was previously available in the United States,[15] but has since been discontinued in this country.[16] Dydrogesterone is also available in elsewhere in Europe, as well as in Central and South America, Asia, and North Africa.[92][3]
References
[edit]References
[edit]- ^ a b c "Dydrogesterone international brands". Drugs.com. Archived from the original on 29 November 2020. Retrieved 29 November 2020.
- ^ a b c d e f g Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 474–. ISBN 978-1-4757-2085-3.
- ^ a b c d e f g h i j k l Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 378–. ISBN 978-3-88763-075-1.
- ^ a b "Femoston 2/10mg film-coated tablets". medicines.ie Ireland. Archived from the original on 2015-04-02. Retrieved 2015-03-16.
- ^ Schindler AE (2015). "Pharmacology of Progestogens". Progestogens in Obstetrics and Gynecology. Springer. pp. 33–40. doi:10.1007/978-3-319-14385-9_2. ISBN 978-3-319-14384-2. S2CID 85844034.
- ^ a b Howard J.A. Carp, MB, BS, FRCOG (9 April 2015). Progestogens in Obstetrics and Gynecology. Springer. pp. 33, 38. ISBN 978-3-319-14385-9.
- ^ a b c d e f g h i j k l m n o p q Kuhl H (August 2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric. 8 (Suppl 1): 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324.
- ^ a b c Beranič N, Gobec S, Rižner TL (May 2011). "Progestins as inhibitors of the human 20-ketosteroid reductases, AKR1C1 and AKR1C3". Chemico-Biological Interactions. 191 (1–3): 227–33. Bibcode:2011CBI...191..227B. doi:10.1016/j.cbi.2010.12.012. PMID 21182831.
- ^ a b c Bińkowska M, Woroń J (June 2015). "Progestogens in menopausal hormone therapy". Przeglad Menopauzalny = Menopause Review. 14 (2): 134–43. doi:10.5114/pm.2015.52154. PMC 4498031. PMID 26327902.
- ^ Olbrich M, Weigl K, Kahler E, Mihara K (October 2016). "Dydrogesterone metabolism in human liver by aldo-keto reductases and cytochrome P450 enzymes". Xenobiotica; the Fate of Foreign Compounds in Biological Systems. 46 (10): 868–74. doi:10.3109/00498254.2015.1134852. PMID 26796435. S2CID 22311056.
- ^ a b Mishell DR, Kirschbaum TH, Morrow CP (May 1990). 1990 The Year Book of Obstetrics and Gynecology. Year Book Medical. ISBN 978-0-8151-6012-0.
- ^ a b "Dydrogesterone/Estradiol (Generic Femoston 1/10mg tablets)". National Health Service (England). 16 August 2018.
- ^ a b c d Schindler AE (December 2009). "Progestational effects of dydrogesterone in vitro, in vivo and on the human endometrium". Maturitas. 65 (Suppl 1): S3-11. doi:10.1016/j.maturitas.2009.10.011. PMID 19969432.
- ^ a b c d e f g h i j k l Tausk MA (1972). "Pharmacology of the Endocrine System and Related Drugs: Progesterone, Progestational Drugs and Antifertility Drugs". International Encyclopaedia of Pharmacology and Therapeutics. 48: 19, 220, 278, 285, 481.
- ^ a b c d e f g h i William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 1411–. ISBN 978-0-8155-1856-3.
- ^ a b Manu P (28 July 2000). The Pharmacotherapy of Common Functional Syndromes: Evidence-Based Guidelines for Primary Care Practice. CRC Press. pp. 235–. ISBN 978-0-7890-0588-5.
The drug is not available for clinical use in the United States.
- ^ Coelingh Bennink HJ, Boerrigter PJ (November 2003). "Use of dydrogesterone as a progestogen for oral contraception". Steroids. 68 (10–13): 927–9. doi:10.1016/j.steroids.2003.07.006. PMID 14667985. S2CID 37470083.
- ^ Balasch J, Vanrell JA, Márquez M, Burzaco I, González-Merlo J (June 1982). "Dehydrogesterone versus vaginal progesterone in the treatment of the endometrial luteal phase deficiency". Fertility and Sterility. 37 (6): 751–4. doi:10.1016/S0015-0282(16)46333-8. PMID 7084497.
- ^ Tomic V (27 January 2014). "Dydrogesterone Versus Intravaginal Progesterone in the Luteal Phase Support". ClinicalTrials.gov.
- ^ Pandian RU (December 2009). "Dydrogesterone in threatened miscarriage: a Malaysian experience". Maturitas. 65 (Suppl 1): S47-50. doi:10.1016/j.maturitas.2009.11.016. PMID 20005647.
- ^ a b Carp H (June 2015). "A systematic review of dydrogesterone for the treatment of recurrent miscarriage". Gynecological Endocrinology. 31 (6): 422–30. doi:10.3109/09513590.2015.1006618. PMID 25765519. S2CID 20795534.
- ^ Tabaste JL, Servaud M, Steiner E, Dabir P, Bene B, Pouzet M (January 1984). "[Action of dydrogesterone in postpubertal menstruation disorders]". Revue Française de Gynécologie et d'Obstétrique. 79 (1): 19–20, 23–5. PMID 6531584.
- ^ a b Dennerstein L, Morse C, Gotts G, Brown J, Smith M, Oats J, et al. (1986). "Treatment of premenstrual syndrome. A double-blind trial of dydrogesterone". Journal of Affective Disorders. 11 (3): 199–205. doi:10.1016/0165-0327(86)90070-4. PMID 2951407.
- ^ Johnston WI (January 1976). "Dydrogesterone and endometriosis". British Journal of Obstetrics and Gynaecology. 83 (1): 77–80. doi:10.1111/j.1471-0528.1976.tb00734.x. PMID 1252380. S2CID 72008984.
- ^ "Dydrogesterone/Estradiol Hormone Replacement Therapy". National Health Service. 16 August 2018.
- ^ a b Trivedi P, Selvaraj K, Mahapatra PD, Srivastava S, Malik S (October 2007). "Effective post-laparoscopic treatment of endometriosis with dydrogesterone". Gynecological Endocrinology. 23 (Suppl 1): 73–6. doi:10.1080/09513590701669583. PMID 17943543. S2CID 23436064.
- ^ Panay N, Pritsch M, Alt J (November 2007). "Cyclical dydrogesterone in secondary amenorrhea: results of a double-blind, placebo-controlled, randomized study". Gynecological Endocrinology. 23 (11): 611–8. doi:10.1080/09513590701582554. PMID 17891596. S2CID 25402423.
- ^ Schweppe KW (December 2009). "The place of dydrogesterone in the treatment of endometriosis and adenomyosis". Maturitas. 65 (Suppl 1): S23-7. doi:10.1016/j.maturitas.2009.11.011. PMID 19945806.
- ^ Winkler UH, Schindler AE, Brinkmann US, Ebert C, Oberhoff C (December 2001). "Cyclic progestin therapy for the management of mastopathy and mastodynia". Gynecological Endocrinology. 15 (Suppl 6): 37–43. doi:10.1080/gye.15.s6.37.43. PMID 12227885. S2CID 27589741.
- ^ Tomic V, Tomic J, Klaic DZ, Kasum M, Kuna K (March 2015). "Oral dydrogesterone versus vaginal progesterone gel in the luteal phase support: randomized controlled trial". European Journal of Obstetrics, Gynecology, and Reproductive Biology. 186 (1): 49–53. doi:10.1016/j.ejogrb.2014.11.002. PMID 25622239.
- ^ Barbosa MW, Valadares NP, Barbosa AC, Amaral AS, Iglesias JR, Nastri CO, et al. (June 2018). "Oral dydrogesterone vs. vaginal progesterone capsules for luteal-phase support in women undergoing embryo transfer: a systematic review and meta-analysis". JBRA Assisted Reproduction. 22 (2): 148–156. doi:10.5935/1518-0557.20180018. PMC 5982562. PMID 29488367.
- ^ Griesinger G, Blockeel C, Sukhikh GT, Patki A, Dhorepatil B, Yang DZ, et al. (December 2018). "Oral dydrogesterone versus intravaginal micronized progesterone gel for luteal phase support in IVF: a randomized clinical trial". Human Reproduction. 33 (12): 2212–2221. doi:10.1093/humrep/dey306. PMC 6238366. PMID 30304457.
- ^ Carp H (December 2012). "A systematic review of dydrogesterone for the treatment of threatened miscarriage". Gynecological Endocrinology. 28 (12): 983–90. doi:10.3109/09513590.2012.702875. PMC 3518297. PMID 22794306.
- ^ Santen RJ, Allred DC, Ardoin SP, Archer DF, Boyd N, Braunstein GD, et al. (July 2010). "Postmenopausal hormone therapy: an Endocrine Society scientific statement". The Journal of Clinical Endocrinology and Metabolism. 95 (7 Suppl 1): s1 – s66. doi:10.1210/jc.2009-2509. PMC 6287288. PMID 20566620.
- ^ Mueck AO, Seeger H, Bühling KJ (December 2009). "Use of dydrogesterone in hormone replacement therapy". Maturitas. 65 (Suppl 1): S51-60. doi:10.1016/j.maturitas.2009.09.013. PMID 19836909.
- ^ Muller A (19 June 1998). European Drug Index: European Drug Registrations (Fourth ed.). CRC Press. pp. 407–. ISBN 978-3-7692-2114-5.
- ^ Chye T, Teng TK, Hseon TE (27 May 2014). Practical Obstetrics And Gynaecology Handbook For O&g Clinicians And General Practitioners (2nd ed.). World Scientific. pp. 704–. ISBN 978-981-4522-96-0.
- ^ "Femoston". NetDoctor.co.uk. 8 October 2019.
- ^ "Questions and Answers About the WHI Postmenopausal Hormone Therapy Trials". Women's Health Initiative.
- ^ a b c d e "DYDROBOON 10mg Film-Coated Tablets". medicines.ie Ireland.[permanent dead link]
- ^ "DYDROGESTERONE". United States National Library of Medicine.
- ^ "Dydrogesterone". DrugBank.
- ^ Reerink EH, Scholer HF, Westerhof P, Querido A, Kassenaar AA, Diczfalusy E, et al. (April 1960). "A new class of hormonally active steroids". Nature. 186 (4719): 168–9. Bibcode:1960Natur.186..168R. doi:10.1038/186168a0. PMID 14436886. S2CID 4189900.
- ^ a b c d e Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, et al. (December 2003). "Classification and pharmacology of progestins". Maturitas. 46 (Suppl 1): S7 – S16. doi:10.1016/j.maturitas.2003.09.014. PMID 14670641.
- ^ a b c d e f g h i j Rižner TL, Brožič P, Doucette C, Turek-Etienne T, Müller-Vieira U, Sonneveld E, et al. (May 2011). "Selectivity and potency of the retroprogesterone dydrogesterone in vitro". Steroids. 76 (6): 607–15. doi:10.1016/j.steroids.2011.02.043. PMID 21376746. S2CID 31609405.
- ^ a b Cabeza M, Heuze Y, Sánchez A, Garrido M, Bratoeff E (February 2015). "Recent advances in structure of progestins and their binding to progesterone receptors". Journal of Enzyme Inhibition and Medicinal Chemistry. 30 (1): 152–9. doi:10.3109/14756366.2014.895719. PMID 24666307. S2CID 10050607.
- ^ Colombo D, Ferraboschi P, Prestileo P, Toma L (January 2006). "A comparative molecular modeling study of dydrogesterone with other progestational agents through theoretical calculations and nuclear magnetic resonance spectroscopy". The Journal of Steroid Biochemistry and Molecular Biology. 98 (1): 56–62. doi:10.1016/j.jsbmb.2005.07.009. PMID 16216490. S2CID 35936384.
- ^ Yasuda K, Sumi GI, Murata H, Kida N, Kido T, Okada H (August 2018). "The steroid hormone dydrogesterone inhibits myometrial contraction independently of the progesterone/progesterone receptor pathway". Life Sciences. 207: 508–515. doi:10.1016/j.lfs.2018.07.004. PMID 29981319. S2CID 51602442.
- ^ Boris A, Stevenson RH, Trmal T (January 1966). "Some studies of the endocrine properties of dydrogesterone". Steroids. 7 (1): 1–10. doi:10.1016/0039-128X(66)90131-0. PMID 5920860.
- ^ Mittal S (12 July 2013). Threatened Miscarriage – ECAB. Elsevier Health Sciences. pp. 42–. ISBN 978-81-312-3233-0.
- ^ a b Endrikat J, Gerlinger C, Richard S, Rosenbaum P, Düsterberg B (December 2011). "Ovulation inhibition doses of progestins: a systematic review of the available literature and of marketed preparations worldwide". Contraception. 84 (6): 549–57. doi:10.1016/j.contraception.2011.04.009. PMID 22078182.
- ^ Eskes TK, Hein PR, Stolte LA, Kars-Villanueva EB, Crone A, Braaksma JT, et al. (April 1970). "Influence of dydrogesterone on the activity of the nonpregnant human uterus". American Journal of Obstetrics and Gynecology. 106 (8): 1235–1241. doi:10.1016/0002-9378(70)90524-7. PMID 5437816.
- ^ a b c d Horsky J, Presl J (6 December 2012). Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. pp. 329–330. ISBN 978-94-009-8195-9.
- ^ Taubert HD (1978). "Luteal phase insufficiency". Female Infertility. Contributions to Gynecology and Obstetrics. Vol. 4. pp. 78–113. doi:10.1159/000401245. ISBN 978-3-8055-2791-0. PMID 679688.
Fig. 17. Lack of hyperthermic effect of retroprogesterone derivative (Trengestone).
- ^ Henzl MR (1978). "Natural and Synthetic Female Sex Hormones". In Yen SS, Jaffe RB (eds.). Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. W.B. Saunders Co. pp. 421–468. ISBN 978-0-7216-9625-6.
- ^ Lauritzen C (1988). "Natürliche und Synthetische Sexualhormone – Biologische Grundlagen und Behandlungsprinzipien" [Natural and Synthetic Sexual Hormones – Biological Basis and Medical Treatment Principles]. In Schneider HP, Lauritzen C, Nieschlag E (eds.). Grundlagen und Klinik der Menschlichen Fortpflanzung [Foundations and Clinic of Human Reproduction] (in German). Walter de Gruyter. pp. 229–306. ISBN 978-3-11-010968-9. OCLC 35483492.
- ^ Yang Z, Hu Y, Zhang J, Xu L, Zeng R, Kang D (February 2017). "Estradiol therapy and breast cancer risk in perimenopausal and postmenopausal women: a systematic review and meta-analysis". Gynecological Endocrinology. 33 (2): 87–92. doi:10.1080/09513590.2016.1248932. PMID 27898258. S2CID 205631264.
- ^ Sturdee DW (August 2013). "Are progestins really necessary as part of a combined HRT regimen?" (PDF). Climacteric. 16 (Suppl 1): 79–84. doi:10.3109/13697137.2013.803311. PMID 23651281. S2CID 21894200. Archived from the original (PDF) on 2017-08-11. Retrieved 2019-09-18.
- ^ Gompel A, Plu-Bureau G (August 2018). "Progesterone, progestins and the breast in menopause treatment". Climacteric. 21 (4): 326–332. doi:10.1080/13697137.2018.1476483. PMID 29852797. S2CID 46922084.
- ^ Stevenson JC, Panay N, Pexman-Fieth C (September 2013). "Oral estradiol and dydrogesterone combination therapy in postmenopausal women: review of efficacy and safety". Maturitas. 76 (1): 10–21. doi:10.1016/j.maturitas.2013.05.018. PMID 23835005.
Dydrogesterone did not increase the risk of VTE associated with oral estrogen (odds ratio (OR) 0.9, 95% CI 0.4–2.3). Other progestogens (OR 3.9, 95% CI 1.5–10.0) were found to further increase the risk of VTE associated with oral estrogen (OR 4.2, 95% CI 1.5–11.6).
- ^ Schneider C, Jick SS, Meier CR (October 2009). "Risk of cardiovascular outcomes in users of estradiol/dydrogesterone or other HRT preparations". Climacteric. 12 (5): 445–53. doi:10.1080/13697130902780853. PMID 19565370. S2CID 45890629.
The adjusted relative risk of developing a VTE tended to be lower for E/D users (OR 0.84; 95% CI 0.37–1.92) than for users of other HRT (OR 1.42; 95% CI 1.10–1.82), compared to non-users.
- ^ Prior JC (December 2015). "Progesterone or progestin as menopausal ovarian hormone therapy: recent physiology-based clinical evidence". Current Opinion in Endocrinology, Diabetes and Obesity. 22 (6): 495–501. doi:10.1097/MED.0000000000000205. PMID 26512775. S2CID 24335817.
- ^ Sayegh R, Awwad JT (2017). "Five Decades of Hormone Therapy Research: The Long, the Short, and the Inconclusive". Essentials of Menopause Management. Springer. pp. 13–43. doi:10.1007/978-3-319-42451-4_2. ISBN 978-3-319-42449-1.
- ^ Jaakkola S, Lyytinen H, Pukkala E, Ylikorkala O (December 2009). "Endometrial cancer in postmenopausal women using estradiol-progestin therapy". Obstetrics and Gynecology. 114 (6): 1197–204. doi:10.1097/AOG.0b013e3181bea950. PMID 19935019. S2CID 39847270.
- ^ a b Neubauer H, Ma Q, Zhou J, Yu Q, Ruan X, Seeger H, et al. (October 2013). "Possible role of PGRMC1 in breast cancer development". Climacteric. 16 (5): 509–13. doi:10.3109/13697137.2013.800038. PMID 23758160. S2CID 29808177.
- ^ Trabert B, Sherman ME, Kannan N, Stanczyk FZ (April 2020). "Progesterone and Breast Cancer". Endocrine Reviews. 41 (2): 320–344. doi:10.1210/endrev/bnz001. PMC 7156851. PMID 31512725.
- ^ "Dydroboon Prescribing Information" (PDF). Ministry of Health (Israel).
- ^ Knörr K, Beller FK, Lauritzen C (17 April 2013). Lehrbuch der Gynäkologie. Springer-Verlag. pp. 214–. ISBN 978-3-662-00942-0.
- ^ Knörr K, Knörr-Gärtner H, Beller FK, Lauritzen C (8 March 2013). Geburtshilfe und Gynäkologie: Physiologie und Pathologie der Reproduktion. Springer-Verlag. pp. 583–. ISBN 978-3-642-95583-9.
- ^ Labhart A (6 December 2012). Clinical Endocrinology: Theory and Practice. Springer Science & Business Media. pp. 554–. ISBN 978-3-642-96158-8.
- ^ Horský J, Presl J (1981). "Hormonal Treatment of Disorders of the Menstrual Cycle". In Horsky J, Presl K (eds.). Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. pp. 309–332. doi:10.1007/978-94-009-8195-9_11. ISBN 978-94-009-8195-9.
- ^ Ufer J (1969). The Principles and Practice of Hormone Therapy in Gynaecology and Obstetrics. de Gruyter. p. 49. ISBN 9783110006148.
17α-Hydroxyprogesterone caproate is a depot progestogen which is entirely free of side actions. The dose required to induce secretory changes in primed endometrium is about 250 mg. per menstrual cycle.
- ^ Pschyrembel W (1968). Praktische Gynäkologie: für Studierende und Ärzte. Walter de Gruyter. pp. 598, 601. ISBN 978-3-11-150424-7.
- ^ Ferin J (September 1972). "Effects, Duration of Action and Metabolism in Man". In Tausk M (ed.). Pharmacology of the Endocrine System and Related Drugs: Progesterone, Progestational Drugs and Antifertility Agents. Vol. II. Pergamon Press. pp. 13–24. ISBN 978-0080168128. OCLC 278011135.
- ^ Henzl MR, Edwards JA (10 November 1999). "Pharmacology of Progestins: 17α-Hydroxyprogesterone Derivatives and Progestins of the First and Second Generation". In Sitruk-Ware R, Mishell DR (eds.). Progestins and Antiprogestins in Clinical Practice. Taylor & Francis. pp. 101–132. ISBN 978-0-8247-8291-7.
- ^ Brotherton J (1976). Sex Hormone Pharmacology. Academic Press. p. 114. ISBN 978-0-12-137250-7.
- ^ Sang GW (April 1994). "Pharmacodynamic effects of once-a-month combined injectable contraceptives". Contraception. 49 (4): 361–385. doi:10.1016/0010-7824(94)90033-7. PMID 8013220.
- ^ Toppozada MK (April 1994). "Existing once-a-month combined injectable contraceptives". Contraception. 49 (4): 293–301. doi:10.1016/0010-7824(94)90029-9. PMID 8013216.
- ^ Goebelsmann U (1986). "Pharmacokinetics of Contraceptive Steroids in Humans". In Gregoire AT, Blye RP (eds.). Contraceptive Steroids: Pharmacology and Safety. Springer Science & Business Media. pp. 67–111. doi:10.1007/978-1-4613-2241-2_4. ISBN 978-1-4613-2241-2.
- ^ Becker H, Düsterberg B, Klosterhalfen H (1980). "[Bioavailability of cyproterone acetate after oral and intramuscular application in men (author's transl)]" [Bioavailability of Cyproterone Acetate after Oral and Intramuscular Application in Men]. Urologia Internationalis. 35 (6): 381–385. doi:10.1159/000280353. PMID 6452729.
- ^ Moltz L, Haase F, Schwartz U, Hammerstein J (May 1983). "[Treatment of virilized women with intramuscular administration of cyproterone acetate]" [Efficacy of Intra muscularly Applied Cyproterone Acetate in Hyperandrogenism]. Geburtshilfe und Frauenheilkunde. 43 (5): 281–287. doi:10.1055/s-2008-1036893. PMID 6223851.
- ^ Wright JC, Burgess DJ (29 January 2012). Long Acting Injections and Implants. Springer Science & Business Media. pp. 114–. ISBN 978-1-4614-0554-2.
- ^ Chu YH, Li Q, Zhao ZF (April 1986). "Pharmacokinetics of megestrol acetate in women receiving IM injection of estradiol-megestrol long-acting injectable contraceptive". The Chinese Journal of Clinical Pharmacology.
The results showed that after injection the concentration of plasma MA increased rapidly. The meantime of peak plasma MA level was 3rd day, there was a linear relationship between log of plasma MA concentration and time (day) after administration in all subjects, elimination phase half-life t1/2β = 14.35 ± 9.1 days.
- ^ Runnebaum BC, Rabe T, Kiesel L (6 December 2012). Female Contraception: Update and Trends. Springer Science & Business Media. pp. 429–. ISBN 978-3-642-73790-9.
- ^ Artini PG, Genazzani AR, Petraglia F (11 December 2001). Advances in Gynecological Endocrinology. CRC Press. pp. 105–. ISBN 978-1-84214-071-0.
- ^ King TL, Brucker MC, Kriebs JM, Fahey JO (21 October 2013). Varney's Midwifery. Jones & Bartlett Publishers. pp. 495–. ISBN 978-1-284-02542-2.
- ^ a b Carp HJ (2015). "Recurrent Pregnancy Loss. Causes, Controversies, and Treatment". Second Edition.
- ^ a b Die Gestagene. Springer-Verlag. 27 November 2013. pp. 10–, 275–276. ISBN 978-3-642-99941-3.
- ^ Queisser-Luft A (June 2009). "Dydrogesterone use during pregnancy: overview of birth defects reported since 1977". Early Human Development. 85 (6): 375–7. doi:10.1016/j.earlhumdev.2008.12.016. PMID 19193503.
- ^ Freedman W (1986). International Products Liability. Kluwer Law Book Publishers. ISBN 978-0-930273-10-1.
Duphaston was removed from the market in 1979 or about two years after the FDA required the defendant to place warnings on the product.
- ^ "FDA Approved Drugs". U.S. Food & Drug Administration.
- ^ a b c d e f Dydrogesterone – Drugs.com
- ^ Göretzlehner G, Lauritzen C, Römer T, Rossmanith W (1 January 2012). Praktische Hormontherapie in der Gynäkologie. Walter de Gruyter. pp. 148–. ISBN 978-3-11-024568-4.
Further reading
[edit]- Foster RH, Balfour JA (October 1997). "Estradiol and dydrogesterone. A review of their combined use as hormone replacement therapy in postmenopausal women". Drugs & Aging. 11 (4): 309–32. doi:10.2165/00002512-199711040-00006. PMID 9342560. S2CID 1733575.
- Gruber CJ, Huber JC (December 2005). "The role of dydrogesterone in recurrent (habitual) abortion". The Journal of Steroid Biochemistry and Molecular Biology. 97 (5): 426–30. doi:10.1016/j.jsbmb.2005.08.009. PMID 16188436. S2CID 25237037.
- Seeger H, Mueck AO (October 2007). "Effects of dydrogesterone on the vascular system". Gynecological Endocrinology. 23 (Suppl 1): 2–8. doi:10.1080/09513590701584998. PMID 17943533. S2CID 13380251.
- Simoncini T, Mannella P, Pluchino N, Genazzani AR (October 2007). "Comparative effects of dydrogesterone and medroxyprogesterone acetate in critical areas: the brain and the vessels". Gynecological Endocrinology. 23 (Suppl 1): 9–16. doi:10.1080/09513590701585094. PMID 17943534. S2CID 21885370.
- Queisser-Luft A (June 2009). "Dydrogesterone use during pregnancy: overview of birth defects reported since 1977". Early Human Development. 85 (6): 375–7. doi:10.1016/j.earlhumdev.2008.12.016. PMID 19193503.
- Mueck AO, Seeger H, Bühling KJ (December 2009). "Use of dydrogesterone in hormone replacement therapy". Maturitas. 65 (Suppl 1): S51-60. doi:10.1016/j.maturitas.2009.09.013. PMID 19836909.
- Schindler AE (December 2009). "Progestational effects of dydrogesterone in vitro, in vivo and on the human endometrium". Maturitas. 65 (Suppl 1): S3-11. doi:10.1016/j.maturitas.2009.10.011. PMID 19969432.
- Schindler AE (February 2011). "Dydrogesterone and other progestins in benign breast disease: an overview". Archives of Gynecology and Obstetrics. 283 (2): 369–71. doi:10.1007/s00404-010-1456-7. PMID 20383772. S2CID 9125889.
- Carp H (December 2012). "A systematic review of dydrogesterone for the treatment of threatened miscarriage". Gynecological Endocrinology. 28 (12): 983–90. doi:10.3109/09513590.2012.702875. PMC 3518297. PMID 22794306.
- Stevenson JC, Panay N, Pexman-Fieth C (September 2013). "Oral estradiol and dydrogesterone combination therapy in postmenopausal women: review of efficacy and safety". Maturitas. 76 (1): 10–21. doi:10.1016/j.maturitas.2013.05.018. PMID 23835005.
- Carp H (June 2015). "A systematic review of dydrogesterone for the treatment of recurrent miscarriage". Gynecological Endocrinology. 31 (6): 422–30. doi:10.3109/09513590.2015.1006618. PMID 25765519. S2CID 20795534.
- Barbosa MW, Silva LR, Navarro PA, Ferriani RA, Nastri CO, Martins WP (August 2016). "Dydrogesterone vs progesterone for luteal-phase support: systematic review and meta-analysis of randomized controlled trials". Ultrasound in Obstetrics & Gynecology. 48 (2): 161–70. doi:10.1002/uog.15814. PMID 26577241.
- Mirza FG, Patki A, Pexman-Fieth C (2016). "Dydrogesterone use in early pregnancy". Gynecological Endocrinology. 32 (2): 97–106. doi:10.3109/09513590.2015.1121982. PMID 26800266. S2CID 21807283.
- Hudic I, Schindler AE, Szekeres-Bartho J, Stray-Pedersen B (September 2016). "Dydrogesterone and pre-term birth". Hormone Molecular Biology and Clinical Investigation. 27 (3): 81–3. doi:10.1515/hmbci-2015-0064. PMID 26812800. S2CID 43183154.
- Raghupathy R, Szekeres-Bartho J (August 2016). "Dydrogesterone and the immunology of pregnancy". Hormone Molecular Biology and Clinical Investigation. 27 (2): 63–71. doi:10.1515/hmbci-2015-0062. PMID 26812877. S2CID 45093373.
- Mohamad Razi ZR, Schindler AE (August 2016). "Review on role of progestogen (dydrogesterone) in the prevention of gestational hypertension". Hormone Molecular Biology and Clinical Investigation. 27 (2): 73–6. doi:10.1515/hmbci-2015-0070. PMID 27101553. S2CID 24715919.
- Schindler AE (August 2016). "Present and future aspects of dydrogesterone in prevention or treatment of pregnancy disorders: an outlook". Hormone Molecular Biology and Clinical Investigation. 27 (2): 49–53. doi:10.1515/hmbci-2016-0028. PMID 27662647. S2CID 23101112.
- Lee HJ, Park TC, Kim JH, Norwitz E, Lee B (2017). "The Influence of Oral Dydrogesterone and Vaginal Progesterone on Threatened Abortion: A Systematic Review and Meta-Analysis". BioMed Research International. 2017: 3616875. doi:10.1155/2017/3616875. PMC 5748117. PMID 29392134.
- Griesinger G, Blockeel C, Tournaye H (May 2018). "Oral dydrogesterone for luteal phase support in fresh in vitro fertilization cycles: a new standard?". Fertility and Sterility. 109 (5): 756–762. doi:10.1016/j.fertnstert.2018.03.034. PMID 29778368.
- Griesinger G, Tournaye H, Macklon N, Petraglia F, Arck P, Blockeel C, et al. (February 2019). "Dydrogesterone: pharmacological profile and mechanism of action as luteal phase support in assisted reproduction". Reproductive Biomedicine Online. 38 (2): 249–259. doi:10.1016/j.rbmo.2018.11.017. PMID 30595525.
