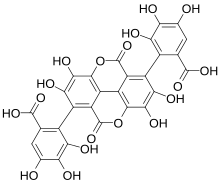
From Wikipedia, the free encyclopedia
Gallagic acid
Names
Preferred IUPAC name
2,2′-(1,2,6,7-Tetrahydroxy-4,9-dioxo-4,9-dihydro-[1]benzopyrano[5,4,3-cde ][1]benzopyran-3,8-diyl)bis(3,4,5-trihydroxybenzoic acid)
Identifiers
ChemSpider
UNII
InChI=1S/C28H14O18/c29-5-1-3(25(39)40)7(17(33)15(5)31)9-13-11-12-14(28(44)46-23(11)21(37)19(9)35)10(20(36)22(38)24(12)45-27(13)43)8-4(26(41)42)2-6(30)16(32)18(8)34/h1-2,29-38H,(H,39,40)(H,41,42)
Key: ZASJRRFAYSNSHU-UHFFFAOYSA-N
Oc4c6c1c(c5c(=O)o6)c(oc(=O)c1c(c4O)-c(c(O)c2O)c(C(O)=O)cc2O)c(O)c(O)c5-c3c(C(O)=O)cc(O)c(O)c3O
Properties
C28 H14 O18
Molar mass
638.39 g/mol
Related compounds
Related compounds
Gallagic acid dilactone ; gallagyldilactone ; terminalin
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Chemical compound
Gallagic acid is a polyphenolic chemical compound that can be found in the ellagitannins, a type of tannin, found in Punica granatum [ 1] punicalagin , punicalin , punicacortein C and 2-O-galloyl-punicalin .
^ Antioxidant, antimalarial and antimicrobial activities of tannin-rich fractions, ellagitannins and phenolic acids from Punica granatum L. Reddy Muntha K., Gupta Sashi K., Jacob Melissa R., Khan Shabana I. and Ferreira Daneel, Planta medica, 2007, vol. 73, no5, pp. 461-467, INIST 18773247
Moieties Lactones Monomers
Acetonyl geraniin Alnusiin Bicornin Carlesiin Casuarictin Emblicanin A and B Euscaphinin Galloyl pedunculagin Grandinin Helioscopinin B Jolkinin Lagerstannin A , B and C Macranganin Myrobalanitannin Nupharin A , B , C , D , E and F Pedunculagin Punicalagin Punigluconin Phyllanemblinin A , B , C , D , E and F Punicalin Roburin E Rugosin E Sanguiin H-5 Stenophyllanin A , B and C Strictinin Tellimagrandin I and II Teracatain Terchebulin Terflavin A and B Tergallic acid Tergallic acid dilactone
Oligomers Other