Organocalcium chemistry
Organocalcium chemistry is the chemistry of compounds containing a calcium to carbon bond,[1] or in broader definitions, organic compounds that contain calcium.[2] Although discovered around the same time as the now commonly utilized organomagnesium compounds,[3] organocalcium compounds were subject to greatly reduced interest due to drastic differences in stability. However, recent advances in stabilization of these highly reactive compounds has spurred increased interest in organocalcium compounds and allowed for multiple research directions to form. Because calcium metal is less reactive to organic reagents than magnesium[4] and the organocalcium compounds are more reactive than organomagnesium compounds, synthesis of novel compounds still poses a significant challenge. Calcium also has access to empty d orbitals that the lighter alkaline earth metals cannot access, and the degree to which this affects bonding and reactivity has sparked a fundamental debate.[5][6] Lastly, despite the inherent instability of most organocalcium complexes, the unique basicity and size of the calcium ion together with the highly polarized bonds formed has opened up applications for organocalcium compounds in organic transformations and catalytic cycles.
Compounds
[edit]In general, organocalcium synthesis is complicated by relatively unreactive calcium metal (compared to magnesium or the alkali metals due to a high atomization energy)[7][8] and high reactivity of most organocalcium compounds to oxygen, water, and even ethereal solvents.[9] To sustain the highly electropositive calcium center, the vast majority of compounds have anionic ligands by which they can be categorized, with neutral coordinating ligands utilized for increased stability.
Aryl, allyl, and alkyl derivatives
[edit]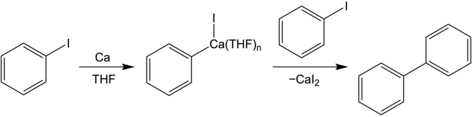
The earliest organocalcium compounds to receive some sustained interest were alkyl- and arylcalcium compounds. The first of these was reported in 1905 by Ernst Beckmann, where synthesis of phenylcalcium iodide was claimed following stirring of calcium shavings with iodobenzene in diethyl ether (Et2O).[3] Subsequent study by Henry Gilman and Ferdinand Schulze argued that the isolated product in this report was actually the Et2O adduct of CaI2,[10] and, although phenylcalcium halides have been reported numerous times,[11][12][13] they are usually characterized through subsequent derivatization products. It took a full century until, in 2005, Matthias Westerhausen and colleagues obtained the first structural characterization of an arylcalcium compound, crystallizing phenylcalcium iodide as an adduct of tetrahydrofuran (THF) and calcium oxide.[14] A consistent challenge in the formation of organocalcium compounds has been the activation of calcium metal. Recent advancements in mechanochemistry have opened up simpler synthetic setups, with unactivated calcium being used to form arylcalcium reagents in situ during ball-milling.[15]
Allylcalcium compounds have also seen recent synthetic success, beginning with Timothy Hanusa and colleagues’ synthesis of a bis(allyl)calcium complex stabilized by sterically large, silyl substituents.[16] These successes have largely been driven by the use of salt metathesis reactions, where potassium salts of allyl anions exchange metals with a calcium halide, typically CaI2. This same strategy has been used to synthesize the unsubstituted complex Ca(η3-C3H5)2 as a soluble triglyme adduct.[17] This has been proven to be a versatile strategy, with a full series of substituted allylcalcium complexes of different sizes also characterized through a salt metathesis pathway.[18]
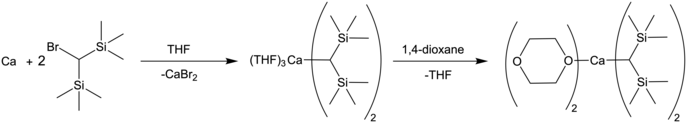
The carbon atom in the calcium-carbon bond takes on a significant negative charge. Because of the greater nucleophilicity of alkyl ligands, the alkylcalcium reagents are in general harder to synthesize than the arylcalcium compounds.[8] A common stabilizing strategy is to use bulky silyl[19] and phenyl[20] substituents to stabilize this negative charge. When targeting a Grignard analogue, the decreased reactivity from this method and the poor stability of the less protected methyl- and ethylcalcium halides has led to in situ generation of reactive alkylcalcium halides as the preferred method over the synthesis of isolable compounds.[21] Because of this poor stability, the pure organometallic dimethylcalcium was only isolated in 2018 by Reiner Anwander and colleagues as an insoluble, amorphous solid, with the THF adduct being structurally characterizable as a heptametallic cluster.[22]
Metallocenes
[edit]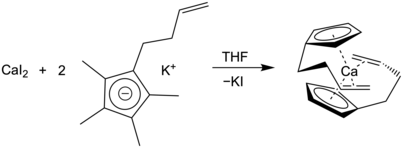
Few calcium metallocenes (“calcocenes”) have been isolated, but they are of particular interest due to the insights into bonding that have come from their study.[23] The first synthesis of Cp2Ca (Cp = cyclopentadienyl) from calcium metal and cyclopentadiene in THF produced an insoluble, polymeric product.[24] A crystal structure showed that, unlike most transition metal metallocenes, the Cp-Ca-Cp angle is significantly bent and Cp2Ca has an opening that can be utilized to access derivatives. As seen in the first monomeric synthesis of a calcocene, ethereal solvents such as Et2O and THF almost always coordinate in this opening and can be challenging to remove through sublimation.[25] This bent structure can be leveraged into different coordination environments. For example, two butenyl-substituted Cp ligand will coordinate to Ca through both the five-membered rings and the olefins, but the olefins will not coordinate to Mg, where the Cp-Mg-Cp angle is not bent.[26]
Low-oxidation-state compounds
[edit]Although low oxidation state beryllium and magnesium chemistry has developed significantly in the last two decades,[27] only a few reports exist of organocalcium compounds stabilizing any oxidation state other than Ca(II).[28] The first and only report of an isolable Ca(I) compound came in 2009, where two THF-coordinated Ca(I) ions sit on either side of an arene ring.[29] The π-antibonding orbitals of the sandwiched arene help stabilize the two calcium ions, which are further stabilized by the coordinating solvent. Other studies of Ca(I) were done at low temperatures in exotic conditions[30] or examine formally Ca(II) compounds that imply Ca(I)-containing intermediates either during synthesis or further reactivity.[31][32] A landmark example of this from Sjoerd Harder and coworkers is the reported reduction of arenes and N2 by a bridged Ca(I)-Ca(I) species generated in situ.[31] The ease of activating the normally inert N2 to turn it into a strong reductant even at room temperature highlights the instability of Ca(I) species. Although not isolable as a Ca(I)-Ca(I) dimer, it possesses similar reactivity as a stronger reducing agent than a Mg(I) dimer.

Amides, hydrides, and fluorides
[edit]There are several classes of calcium complexes that have become especially relevant despite not necessarily containing a Ca-C bond. The calcium amides, for example, have been investigated for numerous applications as a stoichiometric or catalytic reagent. Several modern synthetic strategies have allowed for a wide range of calcium amides to realized. Transmetalation, such as from a Sn(II) amide, allowed for the early preparation of amides yet again stabilized by bulky silyl groups.[33] Additional electronic and kinetic stabilization can be provided through carbenes, despite lacking the π-backbonding that other main group elements are capable of.[34][35] A breakthrough in eliminating side product formation and other contamination was the development of mechanochemical syntheses that forgo the use of solvent. Simply ball-milling CaI2 with a potassium amide salt yielded the corresponding bis(amido) complex.[36]
Inspired by the well-studied and useful solid-state CaH2, several molecular calcium hydrides have been synthesized with the hope of interesting small molecule activation. In 2006, Sjoerd Harder and Julie Brettar accomplished the synthesis of a well-defined, dimeric calcium hydride through the reaction of a calcium amide with phenylsilane.[37] Subsequent studies have expanded the library of stabilizing ligands, but all are multidentate ligands that coordinate through nitrogen sites.[38]
Several recent advances have been made in the synthesis of molecular calcium fluorides. The solid-state CaF2 is an important source of fluorides for organofluorine compounds, but rely on dangerous HF intermediates.[39] The early well-characterized molecular calcium fluorides are clusters and are formed by reacting CaF2 with large, multidentate ligands.[40] Recent work from Simon Aldridge and coworkers have resulted in more accessible fluoride coordination environments that can act as reagents for nucleophilic fluoride addition to organic compounds.[41][42]
Bonding Descriptions
[edit]The changes in properties going down the alkaline earth group causes calcium to possess qualitatively entirely distinct bonding characteristics than the lighter beryllium and magnesium ions. In particular, calcium is significantly larger, more reducing, and has a much lower electronegativity. This enforces a strong preference for the Ca(II) oxidation state and an essentially ionic bond with carbon, which can be reasonably described as a carbanion in the Ca-C bond.[27]
A key difference in calcium bonding descriptions compared to magnesium and beryllium is the occasional use of the unfilled 3d orbitals to fully explain bonding and structural patterns. For example, the bent nature of calcocene, and the potentially bent geometry of CaH2, can be explained by increased involvement of the 3d orbitals in bonding.[23][43] This has been highly debated, however, with other explanations invoking the polarizability of the larger Ca core[44] and a stabilizing van der Waals interaction between the two ligands.[45] A similar debate[5][6][46] is ongoing regarding the degree of π-backbonding in a Ca(CO)8 complex.[30] Although still controversial, computational studies on the degree of sp-d hybridization[47] have caused some to label Ca as an honorary transition metal.[46]
Reactivity
[edit]Heavy Grignard reactivity
[edit]Organocalcium compounds show some more similarities to organolithium chemistry over organomagnesium compounds. This is largely due to differences in electronegativity, which allow organocalcium compounds to function as a base more often than typical magnesium-based Grignard reagents do.[8] This basicity is exemplified by the facile deprotonation and subsequent cleavage of ethers such as THF.[48]

Another point of differentiation from the magnesium-based Grignard reagents is the higher positive charge localized on the calcium atom, due to the higher degree of ionicity in the Ca-C bond versus the Mg-C bond, which can enable unique reactivity not seen in the lighter alkaline earth compounds. For example, a dimeric Ca alkynide complex was shown to enable the coupling of two anionic alkynides to form an extended, fully double bonded four-carbon chain.[49] The previously mentioned in situ generation of reactive alkylcalcium species has also been successfully used to react with amines to form calcium amides.[21] This reactivity relies on fast ligand exchange of calcium Grignard reagents due to the ionic nature of this bond – the initially formed product is a heteroleptic calcium monoamide monohalide, but ligand exchange quickly forms the full calcium diamide and an insoluble calcium dihalide that drives the Schlenk equilibrium to completion. Non-Grignard alkylcalcium complexes have also shown unique reactivity, such as alkylation of benzene driven by the formation of a calcium hydride.[50]
Catalytic reactivity
[edit]Catalysis with organocalcium compounds has historically been limited due to poor stability. However, significant recent progress has been made in multiple areas of catalytic applications. Inspired by alkali metal-based organometallic compounds use in anionic polymerization, organocalcium compounds have also been investigated as polymerization catalysts.[2] For example, fast polymerization has been seen for polylactide synthesis with excellent selectivity for the isotactic form.[51] This is not only enabled by the previously discussed electronic and electrostatic differences, but also by the larger size of calcium in comparison to the alkali metals or magnesium. The larger size of calcium allows an unusual trigonal prismatic coordination geometry utilized throughout the mechanism.[52] The ionic nature of Ca-C bonding can also be leveraged for living polymerization, as was demonstrated for a stereoselective synthesis of polystyrene.[53]
Catalysis has also been performed using organocalcium compounds for a series of organic transformations. This most prominently includes hydroamination, where numerous viable substrates and modes of selectivity have been demonstrated.[2][54][55] Catalytic activity has also been shown for the analogous hydrophosphination,[56] the hydrogenation of alkene with dihydrogen,[57] regioselective hydrosilylation of conjugated alkenes,[58] and the hydroboration of alkenes, although the role of calcium in the latter mechanism is still debated.[59] The redistribution of arylsilane and hydrosilane groups has also been performed catalytically, relying on the cleavage and reformation of C-Si and Si-H bonds driven by the simultaneous cleavage and reformation of Ca-C and Ca-H bonds.[60][61]
References
[edit]- ^ Massey, A. G. (2000). Main group chemistry. Inorganic chemistry (2nd ed.). Chichester ; New York: Wiley. ISBN 978-0-471-49037-1.
- ^ a b c Harder, Sjoerd (2010-07-14). "From Limestone to Catalysis: Application of Calcium Compounds as Homogeneous Catalysts". Chemical Reviews. 110 (7): 3852–3876. doi:10.1021/cr9003659. ISSN 0009-2665. PMID 20420358.
- ^ a b Beckmann, Ernst (January 1905). "Einige Anwendungen von metallischem Calcium". Berichte der Deutschen Chemischen Gesellschaft. 38 (1): 904–906. doi:10.1002/cber.190503801160. ISSN 0365-9496.
- ^ Wu, Tse Chong; Xiong, Heping; Rieke, Reuben D. (August 1990). "Organocalcium chemistry: preparation and reactions of highly reactive calcium". The Journal of Organic Chemistry. 55 (17): 5045–5051. doi:10.1021/jo00304a016. ISSN 0022-3263.
- ^ a b Koch, Daniel; Chen, Yingqian; Golub, Pavlo; Manzhos, Sergei (2019). "Revisiting π backbonding: the influence of d orbitals on metal–CO bonds and ligand red shifts". Physical Chemistry Chemical Physics. 21 (37): 20814–20821. arXiv:1905.06551. Bibcode:2019PCCP...2120814K. doi:10.1039/C9CP04624K. ISSN 1463-9076. PMID 31515551.
- ^ a b Pan, Sudip; Frenking, Gernot (2020). "Comment on "Revisiting π backbonding: the influence of d orbitals on metal–CO bonds and ligand red shifts" by D. Koch, Y. Chen, P. Golub and S. Manzhos, Phys. Chem. Chem. Phys. , 2019, 21, 20814". Physical Chemistry Chemical Physics. 22 (9): 5377–5379. Bibcode:2020PCCP...22.5377P. doi:10.1039/C9CP05951B. ISSN 1463-9076. PMID 32077870.
- ^ Koch, Alexander; Dufrois, Quentin; Wirgenings, Marino; Görls, Helmar; Krieck, Sven; Etienne, Michel; Pohnert, Georg; Westerhausen, Matthias (2018-11-13). "Direct Synthesis of Heavy Grignard Reagents: Challenges, Limitations, and Derivatization". Chemistry – A European Journal. 24 (63): 16840–16850. doi:10.1002/chem.201803518. ISSN 0947-6539. PMID 30095189.
- ^ a b c Harder, Sjoerd; Langer, Jens (2023-11-07). "Opportunities with calcium Grignard reagents and other heavy alkaline-earth organometallics". Nature Reviews Chemistry. 7 (12): 843–853. doi:10.1038/s41570-023-00548-0. ISSN 2397-3358. PMID 37935796.
- ^ Westerhausen, M (August 2008). "Heavy Grignard reagents—Synthesis and reactivity of organocalcium compounds". Coordination Chemistry Reviews. 252 (15–17): 1516–1531. doi:10.1016/j.ccr.2007.10.023.
- ^ Gilman, Henry; Schulze, Ferdinand (September 1926). "Organocalcium Iodides". Journal of the American Chemical Society. 48 (9): 2463–2467. Bibcode:1926JAChS..48.2463G. doi:10.1021/ja01420a038. ISSN 0002-7863.
- ^ Kawabata, N.; Matsumura, A.; Yamashita, S. (January 1973). "Preparation of organocalcium halides". Tetrahedron. 29 (8): 1069–1071. doi:10.1016/0040-4020(73)80063-8.
- ^ Mochida, K.; Ogawa, H. (February 1983). "Preparation and reactions of solvent-free arylcalcium halides ArCaX (X = F, Cl, Br)". Journal of Organometallic Chemistry. 243 (2): 131–135. doi:10.1016/0022-328X(83)89523-0.
- ^ Mochida, Kunio; Yamanishi, Takayuki (October 1987). "A new method for preparation of organocalcium halides by cocondensation of calcium vapor with solvents". Journal of Organometallic Chemistry. 332 (3): 247–252. doi:10.1016/0022-328X(87)85091-X.
- ^ Fischer, Reinald; Görls, Helmar; Westerhausen, Matthias (December 2005). "Reinvestigation of the synthesis of phenylcalcium iodide and the first structural characterization of a heavy Grignard reagent as [((thf)2CaPhI)3·(thf)CaO] with a central Ca4 tetrahedron". Inorganic Chemistry Communications. 8 (12): 1159–1161. doi:10.1016/j.inoche.2005.09.030.
- ^ Gao, Pan; Jiang, Julong; Maeda, Satoshi; Kubota, Koji; Ito, Hajime (2022-10-10). "Mechanochemically Generated Calcium-Based Heavy Grignard Reagents and Their Application to Carbon–Carbon Bond-Forming Reactions". Angewandte Chemie International Edition. 61 (41): e202207118. doi:10.1002/anie.202207118. hdl:2115/90171. ISSN 1433-7851. PMID 35853830.
- ^ Harvey, Melanie J.; Hanusa, Timothy P.; Young, Jr., Victor G. (1999-01-15). "Synthesis and Crystal Structure of the Bis(allyl)calcium Complex [Ca{C3(SiMe3)2H3}2⋅(thf)2]". Angewandte Chemie International Edition. 38 (1–2): 217–219. doi:10.1002/(SICI)1521-3773(19990115)38:1/2<217::AID-ANIE217>3.0.CO;2-Q. ISSN 1433-7851.
- ^ Jochmann, Phillip; Dols, Thomas S.; Spaniol, Thomas P.; Perrin, Lionel; Maron, Laurent; Okuda, Jun (2009-07-20). "Bis(allyl)calcium". Angewandte Chemie International Edition. 48 (31): 5715–5719. doi:10.1002/anie.200901743. ISSN 1433-7851. PMID 19562813.
- ^ Jochmann, Phillip; Maslek, Stefanie; Spaniol, Thomas P.; Okuda, Jun (2011-04-11). "Allyl Calcium Compounds: Synthesis and Structure of Bis(η 3 -1-alkenyl)calcium". Organometallics. 30 (7): 1991–1997. doi:10.1021/om200012k. ISSN 0276-7333.
- ^ Cloke, F. Geoffrey N.; Hitchcock, Peter B.; Lappert, Michael F.; Lawless, Gerard A.; Royo, Beatriz (1991). "Lipophilic strontium and calcium alkyls, amides and phenoxides; X-ray structures of the crystalline square-planar [{trans-Sr(NR′ 2 ) 2 (µ-1,4-dioxane)}∞] and tetrahedral [CaR 2 (1,4-dioxane) 2 ]; R′= SiMe 3 , R = CH(SiMe 3 ) 2 ]". J. Chem. Soc., Chem. Commun. (10): 724–726. doi:10.1039/C39910000724. ISSN 0022-4936.
- ^ McKeever, L.Dennis; Waack, Richard (April 1969). "The electrolytic formation of organometallic compounds". Journal of Organometallic Chemistry. 17 (1): 142–144. doi:10.1016/S0022-328X(00)88045-6.
- ^ a b Schüler, Philipp; Sengupta, Simon; Krieck, Sven; Westerhausen, Matthias (2023-07-14). "In Situ Generation of Magnesium- and Calcium-Based Grignard Reagents for Amide Synthesis". Chemistry – A European Journal. 29 (40): e202300833. doi:10.1002/chem.202300833. ISSN 0947-6539. PMID 37190951.
- ^ Wolf, Benjamin M.; Stuhl, Christoph; Maichle-Mössmer, Cäcilia; Anwander, Reiner (2018-02-14). "Dimethylcalcium". Journal of the American Chemical Society. 140 (6): 2373–2383. Bibcode:2018JAChS.140.2373W. doi:10.1021/jacs.7b12984. ISSN 0002-7863. PMID 29328671.
- ^ a b Hanusa, Timothy P. (2002-06-01). "New Developments in the Cyclopentadienyl Chemistry of the Alkaline-Earth Metals". Organometallics. 21 (13): 2559–2571. doi:10.1021/om020168o. ISSN 0276-7333.
- ^ Zerger, Richard; Stucky, Galen (October 1974). "Unsaturated organometallic compounds of the main group elements. Dicyclopentadienylcalcium". Journal of Organometallic Chemistry. 80 (1): 7–17. doi:10.1016/S0022-328X(00)87011-4.
- ^ Williams, R. Allen; Hanusa, Timothy P.; Huffman, John C. (April 1990). "Structures of ionic decamethylmetallocenes: crystallographic characterization of bis(pentamethylcyclopentadienyl)calcium and -barium and a comparison with related organolanthanide species". Organometallics. 9 (4): 1128–1134. doi:10.1021/om00118a036. ISSN 0276-7333.
- ^ Schumann, Herbert; Schutte, Stefan; Kroth, Heinz-Jürgen; Lentz, Dieter (2004-11-19). "Butenyl-Substituted Alkaline-Earth Metallocenes: A First Step towards Olefin Complexes of the Alkaline-Earth Metals". Angewandte Chemie International Edition. 43 (45): 6208–6211. doi:10.1002/anie.200460927. ISSN 1433-7851. PMID 15549740.
- ^ a b Freeman, Lucas A.; Walley, Jacob E.; Gilliard, Robert J. (2022-06-02). "Synthesis and reactivity of low-oxidation-state alkaline earth metal complexes". Nature Synthesis. 1 (6): 439–448. Bibcode:2022NatSy...1..439F. doi:10.1038/s44160-022-00077-6. ISSN 2731-0582.
- ^ Rösch, Bastian; Harder, Sjoerd (2021). "New horizons in low oxidation state group 2 metal chemistry". Chemical Communications. 57 (74): 9354–9365. doi:10.1039/D1CC04147A. ISSN 1359-7345. PMID 34528959.
- ^ Krieck, Sven; Görls, Helmar; Yu, Lian; Reiher, Markus; Westerhausen, Matthias (2009-03-04). "Stable "Inverse" Sandwich Complex with Unprecedented Organocalcium(I): Crystal Structures of [(thf) 2 Mg(Br)-C 6 H 2 -2,4,6-Ph 3 ] and [(thf) 3 Ca{μ-C 6 H 3 -1,3,5-Ph 3 }Ca(thf) 3 ]". Journal of the American Chemical Society. 131 (8): 2977–2985. doi:10.1021/ja808524y. ISSN 0002-7863. PMID 19193100.
- ^ a b Wu, Xuan; Zhao, Lili; Jin, Jiaye; Pan, Sudip; Li, Wei; Jin, Xiaoyang; Wang, Guanjun; Zhou, Mingfei; Frenking, Gernot (2018-08-31). "Observation of alkaline earth complexes M(CO) 8 (M = Ca, Sr, or Ba) that mimic transition metals". Science. 361 (6405): 912–916. doi:10.1126/science.aau0839. ISSN 0036-8075. PMID 30166489.
- ^ a b Rösch, B.; Gentner, T. X.; Langer, J.; Färber, C.; Eyselein, J.; Zhao, L.; Ding, C.; Frenking, G.; Harder, S. (2021-03-12). "Dinitrogen complexation and reduction at low-valent calcium". Science. 371 (6534): 1125–1128. Bibcode:2021Sci...371.1125R. doi:10.1126/science.abf2374. ISSN 0036-8075. PMID 33707259.
- ^ Liu, Yumiao; Zhu, Kang; Chen, Liang; Liu, Song; Ren, Wenshan (2022-12-19). "Azobenzenyl Calcium Complex: Synthesis and Reactivity Studies of a Ca(I) Synthon". Inorganic Chemistry. 61 (50): 20373–20384. doi:10.1021/acs.inorgchem.2c03008. ISSN 0020-1669. PMID 36475615.
- ^ Westerhausen, Matthias (January 1991). "Synthesis and spectroscopic properties of bis(trimethylsilyl)amides of the alkaline-earth metals magnesium, calcium, strontium, and barium". Inorganic Chemistry. 30 (1): 96–101. doi:10.1021/ic00001a018. ISSN 0020-1669.
- ^ Obi, Akachukwu D.; Freeman, Lucas A.; Coates, Samuel J.; Alexis, Andrew J. H.; Frey, Nathan C.; Dickie, Diane A.; Webster, Charles Edwin; Gilliard, Robert J. (2022-11-14). "Carbene–Calcium Silylamides and Amidoboranes". Organometallics. 41 (21): 3064–3072. doi:10.1021/acs.organomet.2c00464. ISSN 0276-7333.
- ^ Lapshin, Ivan V.; Basalov, Ivan V.; Lyssenko, Konstantin A.; Cherkasov, Anton V.; Trifonov, Alexander A. (2019-01-07). "Ca II , Yb II and Sm II Bis(Amido) Complexes Coordinated by NHC Ligands: Efficient Catalysts for Highly Regio- and Chemoselective Consecutive Hydrophosphinations with PH 3". Chemistry – A European Journal. 25 (2): 459–463. doi:10.1002/chem.201804549. ISSN 0947-6539. PMID 30411413.
- ^ Speight, Isaiah R.; Chmely, Stephen C.; Hanusa, Timothy P.; Rheingold, Arnold L. (2019). "Mechanochemically directed metathesis in group 2 chemistry: calcium amide formation without solvent". Chemical Communications. 55 (15): 2202–2205. doi:10.1039/C8CC10155H. ISSN 1359-7345. PMID 30702108.
- ^ Harder, Sjoerd; Brettar, Julie (2006-05-19). "Rational Design of a Well-Defined Soluble Calcium Hydride Complex". Angewandte Chemie International Edition. 45 (21): 3474–3478. doi:10.1002/anie.200601013. ISSN 1433-7851. PMID 16637088.
- ^ Mukherjee, Debabrata; Schuhknecht, Danny; Okuda, Jun (2018-07-26). "Hydrido Complexes of Calcium: A New Family of Molecular Alkaline-Earth-Metal Compounds". Angewandte Chemie International Edition. 57 (31): 9590–9602. doi:10.1002/anie.201801869. ISSN 1433-7851. PMID 29575506.
- ^ Harsanyi, Antal; Sandford, Graham (2015). "Organofluorine chemistry: applications, sources and sustainability". Green Chemistry. 17 (4): 2081–2086. doi:10.1039/C4GC02166E. ISSN 1463-9262.
- ^ Pevec, Andrej; Demsar, Alojz; Gramlich, Volker; Petricek, Sasa; Roesky, Herbert W. (1997). "Reactions of molecular CaF2 with [(C5Me5)TiF3] and [(C5Me4Et)TiF3]: symbiosis between ionic solids and organometallic compounds". Journal of the Chemical Society, Dalton Transactions (13): 2215–2216. doi:10.1039/a702807e.
- ^ Apolinar, Omar; Struijs, Job J. C.; Sarkar, Debotra; Gouverneur, Véronique; Aldridge, Simon (2024-11-09). "Nucleophilic Fluoride Anion Delivery from Triazacyclononane-Supported Molecular Ca–F Complexes". Angewandte Chemie International Edition: e202414790. doi:10.1002/anie.202414790. ISSN 1433-7851. PMID 39305186.
- ^ Struijs, Job J. C.; Ellwanger, Mathias A.; Crumpton, Agamemnon E.; Gouverneur, Véronique; Aldridge, Simon (September 2024). "Enabling nucleophilic reactivity in molecular calcium fluoride complexes". Nature Chemistry. 16 (9): 1473–1480. Bibcode:2024NatCh..16.1473S. doi:10.1038/s41557-024-01524-x. ISSN 1755-4330. PMC 11375610. PMID 38744913.
- ^ Hayes, Edward F. (November 1966). "Bond Angles and Bonding in Group IIa Metal Dihalides 1a". The Journal of Physical Chemistry. 70 (11): 3740–3742. doi:10.1021/j100883a505. ISSN 0022-3654.
- ^ Guido, M.; Gigli, G. (1976-08-15). "Ion model and equilibrium configuration of the gaseous alkaline-earth dihalides". The Journal of Chemical Physics. 65 (4): 1397–1402. Bibcode:1976JChPh..65.1397G. doi:10.1063/1.433247. ISSN 0021-9606.
- ^ Hollis, T. Keith; Burdett, Jeremy K.; Bosnich, B. (September 1993). "Why are bis(pentamethylcyclopentadienyl) complexes, [MCp2*], of calcium, strontium, barium, samarium, europium, and ytterbium bent?". Organometallics. 12 (9): 3385–3386. doi:10.1021/om00033a003. ISSN 0276-7333.
- ^ a b Zhou, Mingfei; Frenking, Gernot (2021-08-03). "Transition-Metal Chemistry of the Heavier Alkaline Earth Atoms Ca, Sr, and Ba". Accounts of Chemical Research. 54 (15): 3071–3082. doi:10.1021/acs.accounts.1c00277. ISSN 0001-4842. PMID 34264062.
- ^ Voloshina, Elena; Paulus, Beate (2014-04-08). "First Multireference Correlation Treatment of Bulk Metals". Journal of Chemical Theory and Computation. 10 (4): 1698–1706. arXiv:1402.6514. doi:10.1021/ct401040t. ISSN 1549-9618. PMID 26580378.
- ^ Fischer, Reinald; Gärtner, Martin; Görls, Helmar; Yu, Lian; Reiher, Markus; Westerhausen, Matthias (2007-02-26). "THF Solvates of Extremely Soluble Bis(2,4,6-trimethylphenyl)calcium and Tris(2,6-dimethoxyphenyl)dicalcium Iodide". Angewandte Chemie International Edition. 46 (10): 1618–1623. doi:10.1002/anie.200604436. ISSN 1433-7851. PMID 17262875.
- ^ Barrett, Anthony G. M.; Crimmin, Mark R.; Hill, Michael S.; Hitchcock, Peter B.; Lomas, Sarah L.; Procopiou, Panayiotis A.; Suntharalingam, Kogularamanan (2009). "Catalytic 2,3,4-hexatriene formation by terminal alkyne coupling at calcium". Chemical Communications (17): 2299–2301. doi:10.1039/b818848c. ISSN 1359-7345. PMID 19377665.
- ^ Wilson, Andrew S. S.; Hill, Michael S.; Mahon, Mary F.; Dinoi, Chiara; Maron, Laurent (December 2017). "Organocalcium-mediated nucleophilic alkylation of benzene". Science. 358 (6367): 1168–1171. Bibcode:2017Sci...358.1168W. doi:10.1126/science.aao5923. ISSN 0036-8075. PMID 29191904.
- ^ Zhong, Zhiyuan; Schneiderbauer, Stefan; Dijkstra, Pieter J.; Westerhausen, Matthias; Feijen, Jan (2003-11-01). "Single-Site Calcium Initiators for the Controlled Ring-Opening Polymerization of Lactides and Lactones". Polymer Bulletin. 51 (3): 175–182. doi:10.1007/s00289-003-0211-7. ISSN 0170-0839.
- ^ Westerhausen, Matthias; Schneiderbauer, Stefan; Kneifel, Alexander N.; Söltl, Yvonne; Mayer, Peter; Nöth, Heinrich; Zhong, Zhiyuan; Dijkstra, Pieter J.; Feijen, Jan (September 2003). "Organocalcium Compounds with Catalytic Activity for the Ring-Opening Polymerization of Lactones". European Journal of Inorganic Chemistry. 2003 (18): 3432–3439. doi:10.1002/ejic.200300286. ISSN 1434-1948.
- ^ Harder, Sjoerd; Feil, Florian; Knoll, Konrad (2001-11-19). "Novel Calcium Half-Sandwich Complexes for the Living and Stereoselective Polymerization of Styrene". Angewandte Chemie International Edition. 40 (22): 4261–4264. doi:10.1002/1521-3773(20011119)40:22<4261::AID-ANIE4261>3.0.CO;2-J. PMID 29712082.
- ^ Buch, Frank; Harder, Sjoerd (2008-02-01). "A Study on Chiral Organocalcium Complexes: Attempts in Enantioselective Catalytic Hydrosilylation and Intramolecular Hydroamination of Alkenes". Zeitschrift für Naturforschung B. 63 (2): 169–177. doi:10.1515/znb-2008-0209. ISSN 1865-7117.
- ^ Crimmin, Mark R.; Casely, Ian J.; Hill, Michael S. (2005-02-01). "Calcium-Mediated Intramolecular Hydroamination Catalysis". Journal of the American Chemical Society. 127 (7): 2042–2043. Bibcode:2005JAChS.127.2042C. doi:10.1021/ja043576n. ISSN 0002-7863. PMID 15713071.
- ^ Crimmin, Mark R.; Barrett, Anthony G. M.; Hill, Michael S.; Hitchcock, Peter B.; Procopiou, Panayiotis A. (2007-06-01). "Calcium-Catalyzed Intermolecular Hydrophosphination". Organometallics. 26 (12): 2953–2956. doi:10.1021/om070200k. ISSN 0276-7333.
- ^ Spielmann, Jan; Buch, Frank; Harder, Sjoerd (2008-11-24). "Early Main-Group Metal Catalysts for the Hydrogenation of Alkenes with H 2". Angewandte Chemie International Edition. 47 (49): 9434–9438. doi:10.1002/anie.200804657. ISSN 1433-7851. PMID 18979488.
- ^ Buch, Frank; Brettar, Julie; Harder, Sjoerd (2006-04-21). "Hydrosilylation of Alkenes with Early Main-Group Metal Catalysts". Angewandte Chemie International Edition. 45 (17): 2741–2745. doi:10.1002/anie.200504164. ISSN 1433-7851. PMID 16548043.
- ^ Harder, Sjoerd; Spielmann, Jan (February 2012). "Calcium-mediated hydroboration of alkenes: "Trojan horse" or "true" catalysis?". Journal of Organometallic Chemistry. 698: 7–14. doi:10.1016/j.jorganchem.2011.09.025.
- ^ Li, Tao; McCabe, Karl N.; Maron, Laurent; Leng, Xuebing; Chen, Yaofeng (2021-06-04). "Organocalcium Complex-Catalyzed Selective Redistribution of ArSiH 3 or Ar(alkyl)SiH 2 to Ar 3 SiH or Ar 2 (alkyl)SiH". ACS Catalysis. 11 (11): 6348–6356. doi:10.1021/acscatal.1c00463. ISSN 2155-5435.
- ^ Liu, Ruixin; Liu, Xiaojuan; Cheng, Tanyu; Chen, Yaofeng (2022-01-11). "Organocalcium Complex-Catalyzed Dehydrogenative Coupling of Hydrosilanes with Terminal Alkynes". European Journal of Organic Chemistry. 2022 (1). doi:10.1002/ejoc.202101218. ISSN 1434-193X.
