HPTP
Appearance
(Redirected from Draft:HPTP)
 | |
| Clinical data | |
|---|---|
| Drug class | Serotonergic and dopaminergic neurotoxin |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
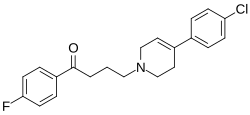
| Formula | C21H21ClFNO |
| Molar mass | 357.85 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
HPTP is a monoaminergic neurotoxin related to MPTP.[1][2][3][4] It is the dehydration product of haloperidol.[1][2][4] The agent is specifically a dopaminergic and serotonergic neurotoxin.[1] HPTP is a prodrug of HPP+, which mediates its monoaminergic neurotoxicity.[1][2][4] This is analogous to how MPP+ mediates the neurotoxicity of MPTP.[1][2][4] Other related compounds include RHPTP and RHPP+.[5]
References
[edit]- ^ a b c d e Kostrzewa RM (2022). "Survey of Selective Monoaminergic Neurotoxins Targeting Dopaminergic, Noradrenergic, and Serotoninergic Neurons". Handbook of Neurotoxicity. Cham: Springer International Publishing. pp. 159–198. doi:10.1007/978-3-031-15080-7_53. ISBN 978-3-031-15079-1.
- ^ a b c d Igarashi, Kazuo (1998). "The Possible Role of an Active Metabolite Derived from the Neuroleptic Agent Haloperidol in Drug-Induced Parkinsonism". Journal of Toxicology: Toxin Reviews. 17 (1): 27–38. doi:10.3109/15569549809006488. ISSN 0731-3837.
- ^ Górska A, Marszałł M, Sloderbach A (October 2015). "[The neurotoxicity of pyridinium metabolites of haloperidol]" [The neurotoxicity of pyridinium metabolites of haloperidol]. Postepy Higieny I Medycyny Doswiadczalnej (in Polish). 69: 1169–1175. doi:10.5604/17322693.1175009 (inactive 1 November 2024). PMID 26561842.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ a b c d Castagnoli N, Castagnoli KP, Van der Schyf CJ, Usuki E, Igarashi K, Steyn SJ, Riker RR (1999). "Enzyme-catalyzed bioactivation of cyclic tertiary amines to form potential neurotoxins". Pol J Pharmacol. 51 (1): 31–38. PMID 10389142.
- ^ Avent KM, DeVoss JJ, Gillam EM (July 2006). "Cytochrome P450-mediated metabolism of haloperidol and reduced haloperidol to pyridinium metabolites". Chem Res Toxicol. 19 (7): 914–920. doi:10.1021/tx0600090. PMID 16841959.
