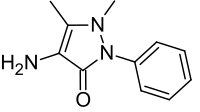
Metamizole
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Novalgin, Algocalmin,[1] Analgin, others[2] |
| Other names | Dipyrone (BAN UK, USAN US), Sulpyrine (JAN JP) |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Oral, IM, IV, rectal |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 100% (active metabolites)[11] |
| Protein binding | 48–58% (active metabolites)[11] |
| Metabolism | Liver[11] |
| Elimination half-life | 14 minutes (parent compound; parenteral);[4] metabolites: 2–4 h[11] |
| Duration of action | 4–6 h[12] |
| Excretion | Urine (96%, IV; 85%, oral), faeces (4%, IV).[4] |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.631 |
| Chemical and physical data | |
| Formula | C13H17N3O4S |
| Molar mass | 311.36 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |

Metamizole or dipyrone is a painkiller, spasm reliever, and fever reliever drug. It is most commonly given by mouth or by intravenous infusion.[13][11][14] It belongs to the ampyrone sulfonate family of medicines and was patented in 1922. Metamizole is marketed under various trade names.[2][3] It was first used medically in Germany under the brand name "Novalgin", [15] later becoming widely known in Slavic nations and India under the name "Analgin".[16][17]
Sale of Metamizole is restricted in some jurisdictions following studies in the 1970s which correlated it to severe adverse effects, including agranulocytosis.[18] Other studies have disputed this judgement, instead claiming that it is a safer drug than other painkillers.[19][20] Metamizole is popular in many countries, where it is typically available as an over-the-counter medication. [21]
Medical uses
[edit]Metamizole, with its potent analgesic (pain relief), antipyretic (fever reduction), and spasmolytic (relax muscle contractions) properties, is utilized in the management of acute pain, fever, and pain caused by muscle spasms.[22]
It is primarily used for perioperative pain, acute injury, colic, cancer pain, other acute/chronic forms of pain and high fever unresponsive to other agents.[4] Metamizole also effectively manages biliary and intestinal colic-like pain, and reduces the spasm of the smooth muscle of the sphincter of Oddi.[23]
Special populations
[edit]Its use in pregnancy is advised against, although animal studies are reassuring in that they show minimal risk of birth defects. Its use in the elderly and those with liver or kidney impairment is advised against, but if these groups of people must be treated, a lower dose and caution is usually advised. Its use during lactation is advised against, as it is excreted in breast milk.[4]
Adverse effects
[edit]While metamizole is a relatively safe medication,[24] it is not entirely devoid of adverse effects.[24]
Metamizole has a potential of blood-related toxicity (blood dyscrasias), but causes less kidney, cardiovascular, and gastrointestinal toxicity than non-steroidal anti-inflammatory drugs (NSAIDs).[11] Like NSAIDs, it can trigger bronchospasm or anaphylaxis, especially in those with asthma.[14]
Serious side effects include agranulocytosis, aplastic anaemia, hypersensitivity reactions (like anaphylaxis and bronchospasm), toxic epidermal necrolysis and it may provoke acute attacks of porphyria, as it is chemically related to the sulfonamides.[3][11][14] The relative risk for agranulocytosis appears to greatly vary according to the country of estimates on said rate and opinion on the risk is strongly divided.[3][25][26] Genetics may play a significant role in metamizole sensitivity.[27] It is suggested that some populations are more prone to suffer from metamizole induced agranulocytosis than others. As an example, metamizole-related agranulocytosis seems to be an adverse effect more frequent in British population as opposed to Spaniards.[28] An assessment report by the European Medicines Agency remarked that "the potential to induce agranulocytosis may be associated with genetic characteristics of the population studied".[29]
A 2015 meta-analysis concluded that on the evidence available "for short-term use in the hospital setting, metamizole seems to be a safe choice when compared to other widely used analgesics", but that the "results were limited by the mediocre overall quality of the reports" analysed.[30]
A systematic review from 2016 found that metamizole significantly increased the relative risk of upper gastrointestinal bleeding, by a factor of 1.4–2.7 times.[31] A study by one of the manufacturers of the drug found the risk of agranulocytosis within the first week of treatment to be a 1.1 in a million, versus 5.9 in a million for diclofenac.[medical citation needed] Therapeutic effect of metamizole on intestinal colic is attributed to its analgesic properties, with no evidence of interference in small bowel or colon motility.[23]
Metamizole has potential for hepatotoxicity.[32]
Contraindications
[edit]Previous hypersensitivity (such as agranulocytosis or anaphylaxis) to metamizole or any of the excipients (e.g. lactose) in the preparation used, acute porphyria, impaired haematopoiesis (such as due to treatment with chemotherapy agents), third trimester of pregnancy (potential for adverse effects in the newborn), lactation, children with a body weight below 16 kg, history of aspirin-induced asthma and other hypersensitivity reactions to analgesics.[4]
In 2018, the European Medicines Agency (EMA) reviewed the safety of metamizole and concluded it to be generally safe for the general population. However, they advised against its use in the third trimester of pregnancy or while breastfeeding due to risks of renal impairment or ductus arteriosus to the fetus or infant.[9]
Interactions
[edit]A clinically severe interaction has been identified between aspirin and metamizole for patients who regularly take aspirin to manage vascular disease: this interaction occurs due to steric hindrance at the active aspirin binding site of COX-1 by metamizole; to manage this interaction, it is recommended to make a delay between the intake of each of these drugs, with aspirin being taken at least 30 minutes before metamizole.[33]
| Drug(s) | Interaction/reason for theoretical potential for interaction |
|---|---|
| Aspirin | Platelet aggregation inhibition (PAI) by aspirin; metamizole prevents aspirin from inhibiting COX-1.[33] |
| Ciclosporin | Decreased serum levels of ciclosporin.[4] |
| Chlorpromazine | Additive hypothermia (low body temperature) may result.[4] |
| Hydroxyethyl starch | Acute renal insufficiency. Low evidence, but discouraged simultaneous administration.[34] |
| Methotrexate | Additive risk for haematologic (blood) toxicity.[4] More specifically, bone marrow aplasia.[34] |
Oral anticoagulants (blood thinners), lithium, captopril, triamterene and antihypertensives may also interact with metamizole, as other pyrazolones are known to interact adversely with these substances.
Overdose
[edit]It is considered fairly safe on overdose, but in these cases supportive measures are usually advised as well as measures to limit absorption (such as activated charcoal) and accelerate excretion (such as haemodialysis).[4]
Physicochemistry
[edit]Metamizole is a sulfonic acid and comes in calcium, sodium and magnesium salt forms.[3] Its sodium salt monohydrate form is a white/almost crystalline powder that is unstable in the presence of light, highly soluble in water and ethanol but practically insoluble in dichloromethane.[35]
Pharmacology
[edit]Its precise mechanism of action of metamizole is unknown, although it is believed that metamizole generally exerts its action by inhibiting the COX-3 enzyme which is responsible for the biosynthesis of prostaglandins in the central nervous system (CNS)—in the brain and spinal cord. Prostaglandins are lipid compounds that play a role in inflammation, pain, and fever. By inhibiting the COX-3 enzyme in the CNS, metamizole reduces the production of prostaglandins, thereby alleviating pain, reducing fever, and potentially lessening inflammation.[14][36] Activation of the endocannabinoid and opioidergic systems is also thought to play a role in analgesic effects of metamizole.[37][20] Metamizole is classified as an atypical nonsteroidal anti-inflammatory drug (NSAID). Unlike typical NSAIDs, metamizole exhibits weak or no anti-inflammatory properties (at least in therapeutic doses),[22][36] but possesses potent analgesic effects via its action in the CNS: this central action distinguishes it from other NSAIDs, which generally exert their effects peripherally.[36] The inhibition of COX-1 and COX-2 by metamizole is less potent than the inhibition of these enzymes by traditional NSAIDs.[12]
Metamizole is metabolized in the liver, where it is converted into active metabolites through the process of N-demethylation.[38] The mechanism of action of metamizole is believed to be exerted via its active metabolites, specifically, arachidonoyl-4-methylaminoantipyrine (ARA-4-MAA) and arachidonoyl-4-aminoantipyrine (ARA-4-AA).[39][19][24] This mechanism of action has been compared to paracetamol and its active arachidonic acid metabolite AM404. The CB1 receptor inverse agonist AM-251 was able to reduce the cataleptic response and thermal analgesia of metamizole.[40] Another study found its antihyperalgesic effect reversed by the CB2 inverse agonist AM-630[41] Although it seems to inhibit fevers caused by prostaglandins, especially prostaglandin E2,[42] metamizole appears to produce its therapeutic effects by means of its metabolites, especially N-methyl-4-aminoantipyrine (MAA) and 4-aminoantipyrine (AA) which form through the FAAH enzyme to create arachidonoyl-4-methylaminoantipyrine (ARA-4-MAA) and arachidonoyl-4-aminoantipyrine (ARA-4-AA).[4]
Metamizole likely induces the CYP2B6 and CYP3A4 enzymes.[22]
| Metabolite | Acronym | Biologically active? | Pharmacokinetic properties |
|---|---|---|---|
| MAA | Yes | Bioavailability≈90%. Plasma protein binding: 58%. Excreted in the urine as 3±1% of the initial (oral) dose | |
| AA | Yes | Bioavailability≈22.5%. Plasma protein binding: 48%. Excreted in the urine as 6±3% of the initial (oral) dose | |
| FAA | No | Plasma protein binding: 18%. Excretion in the urine as 23±4% of the initial oral dose | |
| AAA | No | Plasma protein binding: 14%. Excretion in the urine as 26±8% of the initial oral dose |
History
[edit]Ludwig Knorr was a student of Emil Fischer who won the Nobel Prize for his work on purines and sugars, which included the discovery of phenylhydrazine.[1][43] In the 1880s, Knorr was trying to make quinine derivatives from phenylhydrazine, and instead made a pyrazole derivative, which after a methylation, he made into phenazone, also called antipyrine, which has been called "the 'mother' of all modern antipyretic analgesics."[1][44]: 26–27 Sales of that drug exploded, and in the 1890s chemists at Teerfarbenfabrik Meister, Lucius & Co. (a precursor of Hoechst AG which is now Sanofi), made another derivative called pyramidon which was three times more active than antipyrine.[1]
In 1893, a derivative of antipyrine, aminopyrine, was made by Friedrich Stolz at Hoechst.[44]: 26–27 Yet later, chemists at Hoechst made a derivative, melubrine (sodium antipyrine aminomethanesulfonate), which was introduced in 1913;[45] finally in 1920, metamizole was synthesized.[20] Metamizole is a methyl derivative of melubrine and is also a more soluble prodrug of pyramidon.[1][44]: 26–27 Metamizole was first marketed in Germany as "Novalgin" in 1922.[1][20]
Society and culture
[edit]Legal status
[edit]
World map of availability of Metamizole (Dipyrone) Over-the-counter with limited restrictions. Available, but no data on the requirement of prescriptions. Prescription-only, with fairly limited restrictions on its use. Prescription-only, with extensive restrictions on its use. Banned for human use. It may still be used by veterinary cases in some countries. No data. |
Metamizole is banned in several countries, available by prescription in others (sometimes with strong warnings, sometimes without), and available over the counter in yet others.[18][46][47] For example, approval was withdrawn in Sweden (1974), the US (1977),[48] and India (2013, ban lifted in 2014).[49][17][50]
Although metamizole is banned in the US because of its risk of agranulocytosis,[51] it was reported by small surveys that 28% of Hispanics in Miami have possession of it,[52] and 38% of Hispanics in San Diego reported some usage.[53]
There were unauthorized sales and use of metamizole in horses in the US. After reviewing trial data on its safety, the FDA approved it for treating fever in equines.[8]
Amid the opioid crisis, a study pointed out that the legal status of metamizole has a relation to the consumption of oxycodone, showing the use of those drugs were inversely correlated. Its use could be beneficial when adjusted for the addictive risk of opioids, especially on limited and controlled use of metamizole.[54] A 2019 Israeli conference also justified the approved status as a preventive to opioid dependence, and metamizole being safer than most analgesics for renal impaired patients.[55]
Metamizole is the most sold medication in São Paulo, Brazil, accounting for 488 tons in 2016.[56] Given this contrasting consumption compared to other countries, the Brazilian Health Regulatory Agency (ANVISA) convened an international panel for evaluating its safety in 2001, and the conclusion was that the benefits substantially outweighed the risks, and imposing restrictions would lead to significant negative consequences to the population.[57][26] It is also highly popular in Latin America overall. In 2022 in Brazil alone over 215 million doses were administered.[58]
The Bulgarian pharmaceutical Sopharma produces it under the brand Analgin, which as of 2014, has been the top-selling analgesic in Bulgaria for over a decade.[16]
In Germany, the drug is the most commonly prescribed painreliever.[54]
In 2012, headache accounts for 70% of its use in Indonesia.[59]
In 2018, investigators in Spain looked into Nolotil (as metamizole is known in Spain) after the death of several British people in Spain. A possible factor in these deaths might have been a side effect of metamizole that can cause agranulocytosis (a lowering of white blood cell count).[60]
Brand names
[edit]Metamizole is the international nonproprietary name, and in countries where it is marketed, it is available under many brand names.[2]
In Romania metamizole is available as the original marketed pharmaceutical product by Zentiva as Algocalmin, as 500 mg immediate release tablets. It's also available as an injection with 1 g of metamizole sodium dissolved in 2 ml of solvent.
In Israel it is sold under the brand name "Optalgin" (Hebrew: אופטלגין), manufactured by Teva.
In Czechia it is available under the brand name "Algifen Neo" in the form of drops containing 500 mg/ml of Metamizole and 5 mg/ml of Pitofenone, manufactured by Teva.
It is known as Sulpyrin and Sulpyrine in South Korea (설피린) and Japan. (スルピリン)[61][62]
Analgin
[edit]Analgin (Russian: Анальгин) is a generic name used in the former USSR pharmacopeia,[63] continuing in use in Slavic nations. A firm in Russia tried unsuccessfully in 2011 to claim the name as their trademark.[64][65] In Bulgaria, Sopharma succeeded in registering Analgin as a trademark in 2004.[66]
Analgin is also the common term used in the Indian pharmacopeia.[67]
References
[edit]- ^ a b c d e f Brune K (December 1997). "The early history of non-opioid analgesics". Acute Pain. 1: 33–40. doi:10.1016/S1366-0071(97)80033-2.
- ^ a b c "Metamizole Annex I - List of nationally authorised medicinal products" (PDF). European Medicines Agency. 2018-06-01. Archived (PDF) from the original on 2022-02-05. Retrieved 2023-08-14.
- ^ a b c d e Martindale W, Sweetman SC, eds. (2009). Martindale: The complete drug reference (36th ed.). London; Chicago: Pharmaceuticale Press. p. 49. ISBN 978-0-85369-840-1.
- ^ a b c d e f g h i j k l "Fachinformation (Zusammenfassung der Merkmale des Arzneimittels) Novaminsulfon injekt 1000 mg Lichtenstein Novaminsulfon injekt 2500 mg Lichtenstein" (PDF). Winthrop Arzneimittel GmbH (in German). Zinteva Pharm GmbH. February 2013. Archived (PDF) from the original on 16 October 2018. Retrieved 19 April 2014.
- ^ "Notice of Amendment: Addition of Metamizole (Dipyrone) to the Prescription Drug List (PDL)". Government of Canada. 2022-07-18. Archived from the original on 2022-08-30. Retrieved 2023-08-04.
- ^ "Recent NZ Gazette Notices Relating to Classification". medsafe.govt.nz. 2020-03-06. Archived from the original on 2023-04-29. Retrieved 2023-08-04.
- ^ "Minutes of the 63rd meeting of the Medicines Classification Committee - 10 Oct 2019". medsafe.govt.nz. 2019-12-04. Archived from the original on 2023-06-10. Retrieved 2023-08-04.
- ^ a b FDA's Center for Veterinary Medicine (November 26, 2019). "Zimeta (dipyrone injection) - Veterinarians". FDA. Archived from the original on 2022-01-17. Retrieved 2023-08-04.
- ^ a b European Medicines Agency (2018-09-17). "Metamizole containing medicinal products - EMA recommends aligning doses of metamizole medicines and their use during pregnancy and breastfeeding". European Medicines Agency. Archived from the original on 2023-02-01. Retrieved 2023-08-04.
- CHMP (2018-12-13). "Metamizole - Assessment report" (PDF). European Medicines Agency (published 2019-03-28). Archived (PDF) from the original on 2022-02-05. Retrieved 2023-08-03.
- "Metamizole Annex II - Scientific conclusions" (PDF). European Medicines Agency. 2019-03-28. Archived (PDF) from the original on 2022-02-05. Retrieved 2023-08-03.
- "Metamizole - Annex III - Amendments to relevant sections of the Product Information" (PDF). European Medicines Agency. 2018-06-01. Archived (PDF) from the original on 2022-02-05. Retrieved 2023-08-03.
- ^ "Risikoinformationen - Metamizol (Novalgin, Berlosin, Novaminsulfon, etc.): BfArM weist auf richtige Indikationsstellung und Beachtung von Vorsichtsmaßnahmen und Warnhinweisen hin" [Risk information - Metamizol (Novalgin, Berlosin, Novaminsulfon, etc.): BfArM points out the correct indication and compliance with precautionary measures and warnings]. Bundesinstitut für Arzneimittel und Medizinprodukte (Federal Institute for Pharmaceuticals and Medical Products). 2009-05-28. Archived from the original on 2023-05-18. Retrieved 2023-08-04.
- ^ a b c d e f g Jage J, Laufenberg-Feldmann R, Heid F (April 2008). "[Medikamente zur postoperativen Schmerztherapie: Bewährtes und Neues. Teil 1: Nichtopioide]" [Drugs for postoperative analgesia: routine and new aspects. Part 1: non-opioids]. Der Anaesthesist (in German). 57 (4): 382–390. doi:10.1007/s00101-008-1326-x. PMID 18351305. S2CID 32814418.
- ^ a b Stromer W, Palladini M (2022). "Metamizole: A comprehensive approach to its benefit-risk profile". Evidence for Self-Medication. 2. doi:10.52778/efsm.22.0153.
- ^ "Fachinformation (Zusammenfassung der Merkmale des Arzneimittels) Novaminsulfon injekt 1000 mg Lichtenstein Novaminsulfon injekt 2500 mg Lichtenstein" (PDF). Winthrop Arzneimittel GmbH (in German). Zinteva Pharm GmbH. February 2013. Archived (PDF) from the original on 16 October 2018. Retrieved 19 April 2014.
- ^ a b c d Brack A, Rittner HL, Schäfer M (March 2004). "Nichtopioidanalgetika zur perioperativen Schmerztherapie" [Non-opioid analgesics for perioperative pain therapy. Risks and rational basis for use]. Der Anaesthesist (in German). 53 (3): 263–280. doi:10.1007/s00101-003-0641-5. PMID 15021958. S2CID 8829564.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 530. ISBN 978-3-527-60749-5.
- ^ a b Petkova V, Valchanova V, Ibrahim A, Nikolova I, Benbasat N, Dimitrov M (March 2014). "Marketing approaches for OTC analgesics in Bulgaria". Biotechnology, Biotechnological Equipment. 28 (2): 360–365. doi:10.1080/13102818.2014.911477. PMC 4433822. PMID 26019521.
- ^ a b "Govt lifts ban on painkiller Analgin". Business Standard India. 19 March 2014. Archived from the original on 7 January 2018. Retrieved 7 January 2018.
- ^ a b United Nations Department of Economic and Social Affairs (2005). Consolidated List of Products Whose Consumption and/or Sale Have Been Banned, Withdrawn, Severely Restricted of Not Approved by Governments (PDF) (12th ed.). New York: United Nations. pp. 171–5. Archived (PDF) from the original on 21 April 2021. Retrieved 3 April 2013.
- ^ a b Lutz M (November 2019). "Metamizole (Dipyrone) and the Liver: A Review of the Literature". Journal of Clinical Pharmacology. 59 (11): 1433–1442. doi:10.1002/jcph.1512. PMID 31433499. S2CID 201118022.
- ^ a b c d Nikolova I, Tencheva J, Voinikov J, Petkova V, Benbasat N, Danchev N (2014). "Metamizole: A Review Profile of a Well-Known "Forgotten" Drug. Part I: Pharmaceutical and Nonclinical Profile". Biotechnology & Biotechnological Equipment. 26 (6): 3329–3337. doi:10.5504/BBEQ.2012.0089. ISSN 1310-2818. S2CID 56205439.
- ^ Sznejder H, Amand C, Stewart A, Salazar R, Scala WA (2022). "Real world evidence of the use of metamizole (dipyrone) by the Brazilian population. A retrospective cohort with over 380,000 patients". Einstein. 20. Sociedade Beneficente Israelita Brasileira Hospital Albert Einstein: eAO6353. doi:10.31744/einstein_journal/2022ao6353. PMC 9060643. PMID 35584441.
- ^ a b c Lampl C, Likar R (December 2014). "[Metamizole (dipyrone): mode of action, drug-drug interactions, and risk of agranulocytosis]". Schmerz (in German). 28 (6): 584–90. doi:10.1007/s00482-014-1490-7. PMID 25199942.
- ^ a b Collares EF, Troncon LE (January 2019). "Effects of dipyrone on the digestive tract". Braz J Med Biol Res. 52 (2): e8103. doi:10.1590/1414-431X20188103. PMC 6328969. PMID 30652827.
- ^ a b c Jasiecka A, Maślanka T, Jaroszewski JJ (2014). "Pharmacological characteristics of metamizole". Polish Journal of Veterinary Sciences. 17 (1): 207–214. doi:10.2478/pjvs-2014-0030. PMID 24724493.
- ^ Pogatzki-Zahn E, Chandrasena C, Schug SA (October 2014). "Nonopioid analgesics for postoperative pain management". Current Opinion in Anesthesiology. 27 (5): 513–519. doi:10.1097/ACO.0000000000000113. PMID 25102238. S2CID 31337982.
- ^ a b Reis SL, Batista AP, Assumpção J, Ramos ER, Colacite J, Souza LF (2022-10-09). "A bibliographic analysis on the use of Dipyrone and Agranulocytosis". Research, Society and Development. 11 (13): e369111335570. doi:10.33448/rsd-v11i13.35570. S2CID 252870061. Archived from the original on 2023-07-19. Retrieved 2023-07-19.
- ^ García-Martín E, Esguevillas G, Blanca-López N, García-Menaya J, Blanca M, Amo G, et al. (September 2015). "Genetic determinants of metamizole metabolism modify the risk of developing anaphylaxis". Pharmacogenetics and Genomics. 25 (9): 462–464. doi:10.1097/FPC.0000000000000157. PMID 26111152.
- ^ Mérida Rodrigo L, Faus Felipe V, Poveda Gómez F, García Alegría J (April 2009). "Agranulocitosis por metamizol: un potencial problema en la población británica" [Agranulocytosis from metamizole: a potential problem for the British population]. Revista Clínica Española (in Spanish). 209 (4): 176–179. doi:10.1016/s0014-2565(09)71310-4. PMID 19457324.
- ^ Assessment report: metamizole-containing medicinal products (PDF) (Report). European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP). 13 December 2018. EMA/143912/2019. Archived (PDF) from the original on 5 February 2022. Retrieved 4 August 2023.
- ^ Kötter T, da Costa BR, Fässler M, Blozik E, Linde K, Jüni P, et al. (13 April 2015). "Metamizole-associated adverse events: a systematic review and meta-analysis". PLOS ONE. 10 (4). Public Library of Science (PLoS): e0122918. Bibcode:2015PLoSO..1022918K. doi:10.1371/journal.pone.0122918. PMC 4405027. PMID 25875821.
- ^ Andrade S, Bartels DB, Lange R, Sandford L, Gurwitz J (October 2016). "Safety of metamizole: a systematic review of the literature". Journal of Clinical Pharmacy and Therapeutics. 41 (5): 459–477. doi:10.1111/jcpt.12422. PMID 27422768. S2CID 24538147.
- ^ Lutz M (November 2019). "Metamizole (Dipyrone) and the Liver: A Review of the Literature". J Clin Pharmacol. 59 (11): 1433–1442. doi:10.1002/jcph.1512. PMID 31433499.
- ^ a b Schmelzer KP, Liebetrau D, Kämmerer W, Meisinger C, Hyhlik-Dürr A (April 2023). "Strategies for Avoiding Typical Drug-Drug Interactions and Drug-Related Problems in Patients with Vascular Diseases". Medicina (Kaunas). 59 (4): 780. doi:10.3390/medicina59040780. PMC 10142821. PMID 37109738.
 This article incorporates text from this source, which is available under the CC BY 4.0 license.
This article incorporates text from this source, which is available under the CC BY 4.0 license.
- ^ a b Aronson JK, ed. (2015). Meyler's side effects of drugs: the international encyclopedia of adverse drug reactions and interactions. Vol. 4 (16th ed.). Amsterdam Boston Heidelberg: Elsevier. pp. 859–862. ISBN 978-0-444-53716-4. OCLC 927102885.
- ^ "Metamizole". European pharmacopoeia (English 8.1 ed.). Council Of Europe: European Directorate for the Quality of Medicines and Healthcare. 2013. p. 3791. ISBN 978-92-871-7527-4.
- ^ a b c Monteiro B, Steagall PV (November 2019). "Antiinflammatory Drugs". The Veterinary Clinics of North America. Small Animal Practice. 49 (6): 993–1011. doi:10.1016/j.cvsm.2019.07.009. PMID 31519356.
Metamizole (dipyrone) is considered an atypical NSAID with weak antiinflammatory properties, but potent analgesic effects that are thought to be related mainly via central inhibition of COX-3 enzyme
- ^ Himcheva I, Stavreva GT, Naydenova E, Bocheva A (2022-10-06). "Involvement of the opioidergic and nociceptinergic systems in the analgesic effects of novel nociceptin analogues after acute and chronic immobilization stress" (PDF). Pharmacia. 69 (4): 935–942. doi:10.3897/pharmacia.69.e89379. ISSN 2603-557X.
- ^ Bachmann F, Duthaler U, Rudin D, Krähenbühl S, Haschke M (July 2018). "N-demethylation of N-methyl-4-aminoantipyrine, the main metabolite of metamizole". European Journal of Pharmaceutical Sciences. 120: 172–180. doi:10.1016/j.ejps.2018.05.003. PMID 29746911.
- ^ Lupu G, Bel L, Andrei S (November 2022). "Pain Management and Analgesics Used in Small Mammals during Post-Operative Period with an Emphasis on Metamizole (Dipyrone) as an Alternative Medication". Molecules. 27 (21): 7434. doi:10.3390/molecules27217434. PMC 9657641. PMID 36364259.
- ^ Crunfli F, Vilela FC, Giusti-Paiva A (March 2015). "Cannabinoid CB1 receptors mediate the effects of dipyrone". Clinical and Experimental Pharmacology & Physiology. 42 (3): 246–255. doi:10.1111/1440-1681.12347. PMID 25430877. S2CID 13598808.
- ^ Gonçalves Dos Santos G, Vieira WF, Vendramini PH, Bassani da Silva B, Fernandes Magalhães S, Tambeli CH, et al. (May 2020). "Dipyrone is locally hydrolyzed to 4-methylaminoantipyrine and its antihyperalgesic effect depends on CB2 and kappa-opioid receptors activation". European Journal of Pharmacology. 874: 173005. doi:10.1016/j.ejphar.2020.173005. PMID 32057719. S2CID 211112059.
- ^ Malvar D, Aguiar FA, Vaz A, Assis DC, de Melo MC, Jabor VA, et al. (August 2014). "Dipyrone metabolite 4-MAA induces hypothermia and inhibits PGE2 -dependent and -independent fever while 4-AA only blocks PGE2 -dependent fever". British Journal of Pharmacology. 171 (15): 3666–3679. doi:10.1111/bph.12717. PMC 4128064. PMID 24712707.
- ^ "Emil Fischer – Biographical". Nobel Committee. Archived from the original on 2017-06-06. Retrieved 2017-06-14.
- ^ a b c Raviña Rubira E (2011). The Evolution of Drug Discovery: From Traditional Medicines to Modern Drugs. Weinheim: Wiley-VCH. ISBN 978-3-527-32669-3.
- ^ "New and Nonofficial Remedies: Melubrine". JAMA. 61 (11): 869. 1913.
- ^ Department of Economic and Social Affairs of the United Nations Secretariat Consolidated List of Products Whose Consumption and/or Sale Have Been Banned, Withdrawn, Severely Restricted or not Approved by Governments Fourteenth Issue (New data only) (January 2005 – October 2008): Pharmaceuticals Archived 2017-03-09 at the Wayback Machine United Nations – New York, 2009
- ^ Rogosch T, Sinning C, Podlewski A, Watzer B, Schlosburg J, Lichtman AH, et al. (January 2012). "Novel bioactive metabolites of dipyrone (metamizol)". Bioorganic & Medicinal Chemistry. 20 (1): 101–107. doi:10.1016/j.bmc.2011.11.028. PMC 3248997. PMID 22172309.
- ^ Gardner S (1977-06-07). Drug products containing dipyrone - Withdrawal of Approval of New Drug Applications (Report). Vol. 42. Federal Register (published 1977-06-17). pp. 30893–4. ISSN 0097-6326. Archived from the original on 2020-09-29. Retrieved 2023-08-04.
This notice withdraws approval of the new drug applications (NDA's) for drug products containing dipyrone. The drug products have been used to reduce fever, but they are not shown to be safe for use. On the basis of new evidence, not contained in the applications or not available until after the applications were approved, evaluated together with the evidence available when the applications were approved, the Commissioner of Food and Drugs finds that such drugs have not been shown to be safe for use upon the basis of which the applications were approved. (...) approval of the NDA's providing for the drug products named above, and all amendments and supplements applying thereto, is withdrawn effective June 27,1977. Shipment in interstate commerce of the above-listed products or of any identical, related, or similar product, not the subject of an approved NDA, will then be unlawful.
- ^ Bhaumik S (July 2013). "India's health ministry bans pioglitazone, metamizole, and flupentixol-melitracen". BMJ. 347: f4366. doi:10.1136/bmj.f4366. PMID 23833116. S2CID 45107003.
- ^ Panda AK (2014-02-13). Drugs Rules - G.S.R. 86(E) 13.02.2014 Revoke GSR378(E) Reg. Suspension of Analgin (PDF) (Report). New Delhi: The Gazette of India. Archived (PDF) from the original on 2023-08-11. Retrieved 2023-08-11.
- ^ Bonkowsky JL, Frazer JK, Buchi KF, Byington CL (June 2002). "Metamizole use by Latino immigrants: a common and potentially harmful home remedy" (PDF). Pediatrics. 109 (6): e98. doi:10.1542/peds.109.6.e98. PMID 12042592. Retrieved 2024-03-15.
- ^ Garcia S, Canoniero M, Lopes G, Soriano AO (September 2006). "Metamizole use among Hispanics in Miami: report of a survey conducted in a primary care setting". Southern Medical Journal. 99 (9): 924–926. doi:10.1097/01.smj.0000233020.68212.8f. PMID 17004525. S2CID 41638378.
- ^ Taylor L, Abarca S, Henry B, Friedman L (September 2001). "Use of Neo-melubrina, a banned antipyretic drug, in San Diego, California: a survey of patients and providers". The Western Journal of Medicine. 175 (3): 159–163. doi:10.1136/ewjm.175.3.159. PMC 1071527. PMID 11527837.
- ^ a b Preissner S, Siramshetty VB, Dunkel M, Steinborn P, Luft FC, Preissner R (2019). "Pain-Prescription Differences - An Analysis of 500,000 Discharge Summaries". Current Drug Research Reviews. 11 (1): 58–66. doi:10.2174/1874473711666180911091846. PMID 30207223. S2CID 52192130.
- ^ Dinavitser N (January 2020). "Dipyrone – a Good Medication with a Bad Reputation". Journal of Medical Toxicology. 16 (1). Rambam Health Care Campus, Haifa, Israel: American College of Medical Toxicology: 75–86. doi:10.1007/s13181-019-00743-w. PMC 6942089. PMID 31721040.
ACMT/IST 2019 American-Israeli Medical Toxicology Conference
- ^ Aragão RB, Semensatto D, Calixto LA, Labuto G (2020). "Pharmaceutical market, environmental public policies and water quality: the case of the São Paulo Metropolitan Region, Brazil". Cadernos de Saúde Pública. 36 (11): e00192319. doi:10.1590/0102-311x00192319. ISSN 1678-4464. PMID 33237204. S2CID 227168554.
- ^ Brazilian Health Regulatory Agency (ANVISA) (24 July 2001). Painel Internacional de Avaliação da Segurança da Dipirona [International Panel for the Evaluation of Dipyrone Safety] (PDF) (Report) (in Portuguese). Brasília. Archived from the original (PDF) on 2009-01-21.
- ^ Biernath A (2023-08-17). "Dipirona: por que é vendida no Brasil, mas proibida nos EUA e em parte da Europa?". BBC News Brasil (in Brazilian Portuguese). Archived from the original on 2023-10-14. Retrieved 2023-09-21.
- ^ Kurniawati M, Ikawati Z, Raharjo B (2012). "The evaluation of metamizole use in some places of pharmacy service in Cilacap county". Jurnal Manajemen Dan Pelayanan Farmasi (Journal of Management and Pharmacy Practice) (in Indonesian). 2 (1): 50–55. Archived from the original on 7 April 2023. Retrieved 15 March 2022.
- ^ "Exclusive: southern spain hospitals in british expat hotspot issue warning for 'lethal' painkiller nolotil". 23 April 2018. Archived from the original on 28 April 2018. Retrieved 27 April 2018.
- ^ Kim CH (2004-08-26). "해열진통제 '설피린' 국내사용 논란" [Controversy over domestic use of antipyretic analgesic Sulpyrin]. 경기신문 [kgnews] (in Korean). Archived from the original on 2023-08-14. Retrieved 2023-08-14.
- ^ Pharmaceutical and Medical Device Regulatory Science Society of Japan (2022). The Japanese Pharmacopoeia (PDF) (18th English ed.). Yakuji Nippo-sha. p. 1760. ISBN 978-4-8408-1589-5. Archived (PDF) from the original on 2023-01-20. Retrieved 2023-08-14.
- ^ Ministry of Health of the USSR (1968). Государственная Фармакопея Союза Советских Социалистических Республик [State Pharmacopoeia of the Union of Soviet Socialist Republics] (in Russian) (10th ed.). Moscow: Медицина [Medicine]. p. 94.
- ^ Ovchinnikov R (2011-12-22). "Анальгин хотят отпустить в одни руки" [Analgin want to let go in one hand]. Известия (in Russian). Archived from the original on 2023-08-12.
- ^ "Pavel Sadovsky comments on trademarks registering for RIA Novosti - Insights". EPAM Law. 2012-02-22. Archived from the original on 2021-02-25. Retrieved 2023-08-14.
- ^ "Sopharma wins TM battle over analgesic drug Analgin". FFBH. 2004-08-05. Archived from the original on 2023-08-14. Retrieved 2023-08-14.
- ^ Ministry of Health and Family Welfare (2007). Indian Pharmacopeia (PDF). Vol. 2. Ghaziabad, India: Indian Pharmacopoeia Commission. p. 117. Archived (PDF) from the original on 2023-07-28. Retrieved 2023-08-13.