Deep vein thrombosis
| Deep vein thrombosis | |
|---|---|
| Other names | Deep venous thrombosis |
 | |
| DVT in the right leg with swelling and redness | |
| Specialty | Various |
| Symptoms | Pain, swelling, redness, enlarged veins in the affected limb[1] |
| Complications | Post-thrombotic syndrome, recurrent VTE[2] |
| Risk factors | Recent surgery, older age, active cancer, obesity, infection, inflammatory diseases, antiphospholipid syndrome, personal history or family history of VTE, injuries, trauma, lack of movement, hormonal birth control, pregnancy and the period following delivery, genetic factors[3][4] |
| Diagnostic method | Ultrasound[5] |
| Differential diagnosis | Cellulitis, ruptured Baker's cyst, hematoma, lymphedema, chronic venous insufficiency, etc. |
| Prevention | Frequent walking, calf exercises, maintaining a healthy body weight, anticoagulants (blood thinners), intermittent pneumatic compression, graduated compression stockings, aspirin[6][7] |
| Treatment | Anticoagulation, catheter-directed thrombolysis |
| Medication | Direct oral anticoagulants, low-molecular-weight heparin, fondaparinux, unfractionated heparin, warfarin |
| Frequency | From 0.8–2.7 per 1000 people per year, but populations in China and Korea are below this range[8] |
Deep vein thrombosis (DVT) is a type of venous thrombosis involving the formation of a blood clot in a deep vein, most commonly in the legs or pelvis.[9][a] A minority of DVTs occur in the arms.[11] Symptoms can include pain, swelling, redness, and enlarged veins in the affected area, but some DVTs have no symptoms.[1]
The most common life-threatening concern with DVT is the potential for a clot to embolize (detach from the veins), travel as an embolus through the right side of the heart, and become lodged in a pulmonary artery that supplies blood to the lungs. This is called a pulmonary embolism (PE). DVT and PE comprise the cardiovascular disease of venous thromboembolism (VTE).[2]
About two-thirds of VTE manifests as DVT only, with one-third manifesting as PE with or without DVT.[12] The most frequent long-term DVT complication is post-thrombotic syndrome, which can cause pain, swelling, a sensation of heaviness, itching, and in severe cases, ulcers.[5] Recurrent VTE occurs in about 30% of those in the ten years following an initial VTE.[3]
The mechanism behind DVT formation typically involves some combination of decreased blood flow, increased tendency to clot, changes to the blood vessel wall, and inflammation.[13] Risk factors include recent surgery, older age, active cancer, obesity, infection, inflammatory diseases, antiphospholipid syndrome, personal history and family history of VTE, trauma, injuries, lack of movement, hormonal birth control, pregnancy, and the period following birth. VTE has a strong genetic component, accounting for approximately 50 to 60% of the variability in VTE rates.[4] Genetic factors include non-O blood type, deficiencies of antithrombin, protein C, and protein S and the mutations of factor V Leiden and prothrombin G20210A. In total, dozens of genetic risk factors have been identified.[4][14]
People suspected of having DVT can be assessed using a prediction rule such as the Wells score. A D-dimer test can also be used to assist with excluding the diagnosis or to signal a need for further testing.[5] Diagnosis is most commonly confirmed by ultrasound of the suspected veins.[5] VTE becomes much more common with age. The condition is rare in children, but occurs in almost 1% of those ≥ age 85 annually.[3] Asian, Asian-American, Native American, and Hispanic individuals have a lower VTE risk than Whites or Blacks.[4][15] Populations in Asia have VTE rates at 15 to 20% of what is seen in Western countries.[16]
Using blood thinners is the standard treatment. Typical medications include rivaroxaban, apixaban, and warfarin. Beginning warfarin treatment requires an additional non-oral anticoagulant, often injections of heparin.[17][18][19]
Prevention of VTE for the general population includes avoiding obesity and maintaining an active lifestyle. Preventive efforts following low-risk surgery include early and frequent walking. Riskier surgeries generally prevent VTE with a blood thinner or aspirin combined with intermittent pneumatic compression.[7]
Signs and symptoms
[edit]
Symptoms classically affect a leg and typically develop over hours or days,[20] though they can develop suddenly or over a matter of weeks.[21] The legs are primarily affected, with 4–10% of DVT occurring in the arms.[11] Despite the signs and symptoms being highly variable,[5] the typical symptoms are pain, swelling, and redness. However, these symptoms might not manifest in the lower limbs of those unable to walk.[22] In those who are able to walk, DVT can reduce one's ability to do so.[23] The pain can be described as throbbing and can worsen with weight-bearing, prompting one to bear more weight with the unaffected leg.[21][24]
Additional signs and symptoms include tenderness, pitting edema (see image), dilation of surface veins, warmth, discoloration, a "pulling sensation", and even cyanosis (a blue or purplish discoloration) with fever.[5][20][21] DVT can also exist without causing any symptoms.[22] Signs and symptoms help in determining the likelihood of DVT, but they are not used alone for diagnosis.[19]
At times, DVT can cause symptoms in both arms or both legs, as with bilateral DVT.[25] Rarely, a clot in the inferior vena cava can cause both legs to swell.[26] Superficial vein thrombosis, also known as superficial thrombophlebitis, is the formation of a blood clot (thrombus) in a vein close to the skin. It can co-occur with DVT and can be felt as a "palpable cord".[20] Migratory thrombophlebitis (Trousseau's syndrome) is a noted finding in those with pancreatic cancer and is associated with DVT.[27]
Potential complications
[edit]A pulmonary embolism (PE) occurs when a blood clot from a deep vein (a DVT) detaches from a vein (embolizes), travels through the right side of the heart, and becomes lodged as an embolus in a pulmonary artery that supplies deoxygenated blood to the lungs for oxygenation.[28] Up to one-fourth of PE cases are thought to result in sudden death.[12] When not fatal, PE can cause symptoms such as sudden onset shortness of breath or chest pain, coughing up blood (hemoptysis), and fainting (syncope).[29][30] The chest pain can be pleuritic (worsened by deep breaths)[29] and can vary based upon where the embolus is lodged in the lungs. An estimated 30–50% of those with PE have detectable DVT by compression ultrasound.[30]
A rare and massive DVT that causes significant obstruction and discoloration (including cyanosis) is phlegmasia cerulea dolens.[31][32] It is life-threatening, limb-threatening, and carries a risk of venous gangrene.[33] Phlegmasia cerulea dolens can occur in the arm but more commonly affects the leg.[34][35] If found in the setting of acute compartment syndrome, an urgent fasciotomy is warranted to protect the limb.[36] Superior vena cava syndrome is a rare complication of arm DVT.[11]
DVT is thought to be able to cause a stroke in the presence of a heart defect. This is called a paradoxical embolism because the clot abnormally travels from the pulmonary circuit to the systemic circuit while inside the heart. The defect of a patent foramen ovale is thought to allow clots to travel through the interatrial septum from the right atrium into the left atrium.[37][38]
-
A CT image with red arrows indicating PE (grey) in the pulmonary arteries (white)
-
A case of phlegmasia cerulea dolens in the left leg
-
A depiction of a patent foramen ovale
Differential diagnosis
[edit]In most suspected cases, DVT is ruled out after evaluation.[39] Cellulitis is a frequent mimic of DVT, with its triad of pain, swelling, and redness.[20] Symptoms concerning for DVT are more often due to other causes, including cellulitis, ruptured Baker's cyst, hematoma, lymphedema, and chronic venous insufficiency.[1] Other differential diagnoses include tumors, venous or arterial aneurysms, connective tissue disorders,[40] superficial vein thrombosis, muscle vein thrombosis, and varicose veins.[41]
Classification
[edit]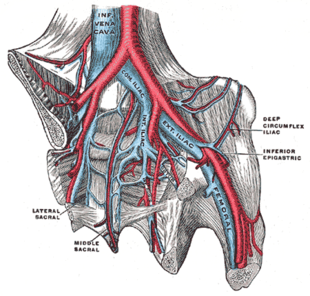
DVT and PE are the two manifestations of the cardiovascular disease venous thromboembolism (VTE).[2] VTE can occur as DVT only, DVT with PE, or PE only.[3] About two-thirds of VTE manifests as DVT only, with one-third manifesting as PE with or without DVT.[12] VTE, along with superficial vein thrombosis, are common types of venous thrombosis.[10]
DVT is classified as acute when the clots are developing or have recently developed, whereas chronic DVT persists more than 28 days.[42] Differences between these two types of DVT can be seen with ultrasound.[43] An episode of VTE after an initial one is classified as recurrent.[44][45] Bilateral DVT refers to clots in both limbs while unilateral means only a single limb is affected.[46]
DVT in a leg above the knee is termed proximal DVT (proximal). DVT in a leg below the knee is termed distal DVT (distal), also called calf DVT when affecting the calf,[47][48] and has limited clinical significance compared to proximal DVT.[49] Calf DVT makes up about half of DVTs.[50] Iliofemoral DVT is described as involving either the iliac, or common femoral vein;[51] elsewhere, it has been defined as involving at a minimum the common iliac vein, which is near the top of the pelvis.[19]
DVT can be classified into provoked and unprovoked categories.[52] For example, DVT that occurs in association with cancer or surgery can be classified as provoked.[52] However, the European Society of Cardiology in 2019 urged for this dichotomy to be abandoned to encourage more personalized risk assessments for recurrent VTE.[53] The distinction between these categories is not always clear.[54]
Causes
[edit]
Traditionally, the three factors of Virchow's triad—venous stasis, hypercoagulability, and changes in the endothelial blood vessel lining—contribute to VTE and were used to explain its formation.[55] More recently, inflammation has been identified as playing a clear causal role.[13] Other related causes include activation of immune system components, the state of microparticles in the blood, the concentration of oxygen, and possible platelet activation.[56] Various risk factors contribute to VTE, including genetic and environmental factors, though many with multiple risk factors never develop it.[57][58]
Acquired risk factors include the strong risk factor of older age,[5] which alters blood composition to favor clotting.[59] Previous VTE, particularly unprovoked VTE, is a strong risk factor.[60] A leftover clot from a prior DVT increases the risk of a subsequent DVT.[61] Major surgery and trauma increase risk because of tissue factor from outside the vascular system entering the blood.[62] Minor injuries,[63] lower limb amputation,[64] hip fracture, and long bone fractures are also risks.[9] In orthopedic surgery, venous stasis can be temporarily provoked by a cessation of blood flow as part of the procedure.[56] Inactivity and immobilization contribute to venous stasis, as with orthopedic casts,[65] paralysis, sitting, long-haul travel, bed rest, hospitalization,[62] catatonia,[66] and in survivors of acute stroke.[67] Conditions that involve compromised blood flow in the veins are May–Thurner syndrome, where a vein of the pelvis is compressed, and venous thoracic outlet syndrome, which includes Paget–Schroetter syndrome, where compression occurs near the base of the neck.[68][69][70]
Infections, including sepsis, COVID-19, HIV, and active tuberculosis, increase risk.[71][72][73][74][75] Chronic inflammatory diseases and some autoimmune diseases,[76] such as inflammatory bowel disease,[77] systemic sclerosis,[78] Behçet's syndrome,[79] primary antiphospholipid syndrome,[80] and systemic lupus erythematosus (SLE)[81] increase risk. SLE itself is frequently associated with secondary antiphospholipid syndrome.[82]
Cancer can grow in and around veins, causing venous stasis, and can also stimulate increased levels of tissue factor.[83] Cancers of the blood, lung, pancreas, brain, stomach, and bowel are associated with high VTE risk.[84] Solid tumors such as adenocarcinomas can contribute to both VTE and disseminated intravascular coagulation. In severe cases, this can lead to simultaneous clotting and bleeding.[85] Chemotherapy treatment also increases risk.[86] Obesity increases the potential of blood to clot, as does pregnancy. In the postpartum, placental tearing releases substances that favor clotting. Oral contraceptives[b] and hormonal replacement therapy increase the risk through a variety of mechanisms, including altered blood coagulation protein levels and reduced fibrinolysis.[56]

Dozens of genetic risk factors have been identified,[14] and they account for approximately 50 to 60% of the variability in VTE rates.[4] As such, family history of VTE is a risk factor for a first VTE.[88] Factor V Leiden, which makes factor V resistant to inactivation by activated protein C,[88] mildly increases VTE risk by about three times.[14][88] Deficiencies of three proteins that normally prevent blood from clotting—protein C, protein S, and antithrombin—contribute to VTE. These deficiencies in antithrombin, protein C, and protein S[c] are rare but strong, or moderately strong, risk factors.[62][56] They increase risk by about 10 times.[89] Having a non-O blood type roughly doubles VTE risk.[56] Non-O blood type is common globally, making it an important risk factor.[90] Individuals without O blood type have higher blood levels of von Willebrand factor and factor VIII than those with O blood type, increasing the likelihood of clotting.[90] Those homozygous for the common fibrinogen gamma gene variant rs2066865 have about a 1.6 times higher risk of VTE.[91] The genetic variant prothrombin G20210A, which increases prothrombin levels,[62] increases risk by about 2.5 times.[14] Additionally, approximately 5% of people have been identified with a background genetic risk comparable to the factor V Leiden and prothrombin G20210A mutations.[14]
Blood alterations including dysfibrinogenemia,[65] low free protein S,[58] activated protein C resistance,[58] homocystinuria,[92] hyperhomocysteinemia,[62] high fibrinogen levels,[62] high factor IX levels,[62] and high factor XI levels[62] are associated with increased risk. Other associated conditions include heparin-induced thrombocytopenia, catastrophic antiphospholipid syndrome,[93] paroxysmal nocturnal hemoglobinuria,[94] nephrotic syndrome,[58] chronic kidney disease,[95] polycythemia vera, essential thrombocythemia,[96] intravenous drug use,[97] and smoking.[d]
Some risk factors influence the location of DVT within the body. In isolated distal DVT, the profile of risk factors appears distinct from proximal DVT. Transient factors, such as surgery and immobilization, appear to dominate, whereas thrombophilias[e] and age do not seem to increase risk.[101] Common risk factors for having an upper extremity DVT include having an existing foreign body (such as a central venous catheter, a pacemaker, or a triple-lumen PICC line), cancer, and recent surgery.[11]
Pathophysiology
[edit]
Blood has a natural tendency to clot when blood vessels are damaged (hemostasis) to minimize blood loss.[102] Clotting is activated by the coagulation cascade and the clearing of clots that are no longer needed is accomplished by the process of fibrinolysis. Reductions in fibrinolysis or increases in coagulation can increase the risk of DVT.[102]
DVT often develops in the calf veins and "grows" in the direction of venous flow, towards the heart.[42][103] DVT most frequently affects veins in the leg or pelvis[9] including the popliteal vein (behind the knee), femoral vein (of the thigh), and iliac veins of the pelvis. Extensive lower-extremity DVT can even reach into the inferior vena cava (in the abdomen).[104] Upper extremity DVT most commonly affects the subclavian, axillary, and jugular veins.[11]
The process of fibrinolysis, where DVT clots can be dissolved back into the blood, acts to temper the process of thrombus growth.[105] This is the preferred process. Aside from the potentially deadly process of embolization, a clot can resolve through organization, which can damage the valves of veins, cause vein fibrosis, and result in non-compliant veins.[106][107] Organization of a thrombus into the vein can occur at the third stage of its pathological development, in which collagen becomes the characteristic component. The first pathological stage is marked by red blood cells, and the second is characterized by medium-textured fibrin.[107]

In arterial thrombosis, blood vessel wall damage is required, as it initiates coagulation,[108] but clotting in the veins mostly occurs without any such mechanical damage.[62] The beginning of venous thrombosis is thought to arise from "activation of endothelial cells, platelets, and leukocytes, with initiation of inflammation and formation of microparticles that trigger the coagulation system" via tissue factor.[77] Vein wall inflammation is likely the inciting event.[77] Importantly, the activated endothelium of veins interacts with circulating white blood cells (leukocytes).[55] While leukocytes normally help prevent blood from clotting (as does normal endothelium), upon stimulation, leukocytes facilitate clotting.[109] Neutrophils are recruited early in the process of venous thrombi formation.[55] They release pro-coagulant granules[109] and neutrophil extracellular traps (NETs) or their components, which play a role in venous thrombi formation.[55][110] NET components are pro-thrombotic through both the intrinsic and extrinsic coagulation pathways.[110] NETs provide "a scaffold for adhesion" of platelets, red blood cells, and multiple factors that potentiate platelet activation.[111] In addition to the pro-coagulant activities of neutrophils, multiple stimuli cause monocytes to release tissue factor.[109] Monocytes are also recruited early in the process.[55]
Tissue factor, via the tissue factor–factor VIIa complex,[112] activates the extrinsic pathway of coagulation and leads to conversion of prothrombin to thrombin, followed by fibrin deposition.[86] Fresh venous clots are red blood cell and fibrin rich.[42] Platelets and white blood cells are also components. Platelets are not as prominent in venous clots as they are in arterial ones, but they can play a role.[56] In cancer, tissue factor is produced by cancer cells.[84] Cancer also produces unique substances that stimulate factor Xa, cytokines that promote endothelial dysfunction, and plasminogen activator inhibitor-1, which inhibits the breakdown of clots (fibrinolysis).[84]

Often, DVT begins in the valves of veins.[105] The blood flow pattern in the valves can cause low oxygen concentrations in the blood (hypoxemia) of a valve sinus. Hypoxemia, which is worsened by venous stasis, activates pathways—ones that include hypoxia-inducible factor-1 and early-growth-response protein 1. Hypoxemia also results in the production of reactive oxygen species, which can activate these pathways, as well as nuclear factor-κB, which regulates hypoxia-inducible factor-1 transcription.[86] Hypoxia-inducible factor-1 and early-growth-response protein 1 contribute to monocyte association with endothelial proteins, such as P-selectin, prompting monocytes to release tissue factor-filled microvesicles, which presumably begin clotting after binding to the endothelial surface.[86]
D-dimers are a fibrin degradation product, a natural byproduct of fibrinolysis that is typically found in the blood. An elevated level[f] can result from plasmin dissolving a clot—or other conditions.[113] Hospitalized patients often have elevated levels for multiple reasons.[39] Anticoagulation, the standard treatment for DVT, prevents further clot growth and PE, but does not act directly on existing clots.[114]
Diagnosis
[edit]A clinical probability assessment using the Wells score (see column in the table below) to determine if a potential DVT is "likely" or "unlikely" is typically the first step of the diagnostic process. The score is used in suspected first lower extremity DVT (without any PE symptoms) in primary care and outpatient settings, including the emergency department.[1][5] The numerical result (possible score −2 to 9) is most commonly grouped into either "unlikely" or "likely" categories.[1][5] A Wells score of two or more means DVT is considered "likely" (about a 28% chance), while those with a lower score are considered "unlikely" to have DVT (about a 6% chance).[39] In those unlikely to have DVT, a diagnosis is excluded by a negative D-dimer blood test.[1] In people with likely DVT, ultrasound is the standard imaging used to confirm or exclude a diagnosis.[5] Imaging is also needed for hospital inpatients with suspected DVT and those initially categorized as unlikely to have DVT but who have a positive D-dimer test.[1]
While the Wells score is the predominant and most studied clinical prediction rule for DVT,[39][115] it does have drawbacks. The Wells score requires a subjective assessment regarding the likelihood of an alternate diagnosis and performs less well in the elderly and those with a prior DVT. The Dutch Primary Care Rule has also been validated for use. It contains only objective criteria but requires obtaining a D-dimer value.[116] With this prediction rule, three points or less means a person is at low risk for DVT. A result of four or more points indicates an ultrasound is needed.[116] Instead of using a prediction rule, experienced physicians can make a DVT pre-test probability assessment using clinical assessment and gestalt, but prediction rules are more reliable.[1]
| Criteria | Wells score for DVT[g] | Dutch Primary Care Rule |
|---|---|---|
| Active cancer (treatment within last 6 months or palliative) | +1 point | +1 point |
| Calf swelling ≥ 3 cm compared to asymptomatic calf (measured 10 cm below tibial tuberosity) | +1 point | +2 points |
| Swollen unilateral superficial veins (non-varicose, in symptomatic leg) | +1 point | +1 point |
| Unilateral pitting edema (in symptomatic leg) | +1 point | — |
| Previous documented DVT | +1 point | — |
| Swelling of entire leg | +1 point | — |
| Localized tenderness along the deep venous system | +1 point | — |
| Paralysis, paresis, or recent cast immobilization of lower extremities | +1 point | — |
| Recently bedridden ≥ 3 days, or major surgery requiring regional or general anesthetic in the past 12 weeks | +1 point | +1 point |
| Alternative diagnosis at least as likely | −2 points | — |
| Positive D-dimer (≥ 0.5 mcg/mL or 1.7 nmol/L) | — | +6 points |
| Absence of leg trauma | — | +1 point |
| Male sex | — | +1 point |
| Use of oral contraceptives | — | +1 point[5][116] |
Compression ultrasonography for suspected deep vein thrombosis is the standard diagnostic method, and it is highly sensitive for detecting an initial DVT.[118] A compression ultrasound is considered positive when the vein walls of normally compressible veins do not collapse under gentle pressure.[39] Clot visualization is sometimes possible, but is not required.[119] Three compression ultrasound scanning techniques can be used, with two of the three methods requiring a second ultrasound some days later to rule out the diagnosis.[118] Whole-leg ultrasound is the option that does not require a repeat ultrasound,[118] but proximal compression ultrasound is frequently used because distal DVT is only rarely clinically significant.[117] Ultrasound methods including duplex and color flow Doppler can be used to further characterize the clot[117] and Doppler ultrasound is especially helpful in the non-compressible iliac veins.[119]
CT scan venography, MRI venography, or a non-contrast MRI are also diagnostic possibilities.[120] The gold standard for judging imaging methods is contrast venography, which involves injecting a peripheral vein of the affected limb with a contrast agent and taking X-rays, to reveal whether the venous supply has been obstructed. Because of its cost, invasiveness, availability, and other limitations, this test is rarely performed.[39]
-
An ultrasound with a blood clot visible in the left common femoral vein. (The common femoral vein is distal to the external iliac vein.)
-
Doppler ultrasonography showing absence of flow and hyperechogenic content in a clotted femoral vein (labeled subsartorial[h]) distal to the branching point of the deep femoral vein. When compared to this clot, clots that instead obstruct the common femoral vein (proximal to this branching point) cause more severe effects due to impacting a significantly larger portion of the leg.[122]
-
An abdominal CT scan demonstrating an iliofemoral DVT, with the clot in the right common iliac vein of the pelvis
-
Vascular anatomy for deep venous thrombosis (DVT) point of care ultrasound (POCUS)
Management
[edit]Treatment for DVT is warranted when the clots are either proximal, distal and symptomatic, or upper extremity and symptomatic.[2] Providing anticoagulation, or blood-thinning medicine, is the typical treatment after patients are checked to make sure they are not subject to bleeding.[2][i] However, treatment varies depending upon the location of DVT. For example, in cases of isolated distal DVT, ultrasound surveillance (a second ultrasound after 2 weeks to check for proximal clots), might be used instead of anticoagulation.[5][124] Although, those with isolated distal DVT at a high risk of VTE recurrence are typically anticoagulated as if they had proximal DVT. Those at a low risk for recurrence might receive a four- to six-week course of anticoagulation, lower doses, or no anticoagulation at all.[5] In contrast, those with proximal DVT should receive at least 3 months of anticoagulation.[5]
Some anticoagulants can be taken by mouth, and these oral medicines include warfarin (a vitamin K antagonist), rivaroxaban (a factor Xa inhibitor), apixaban (a factor Xa inhibitor), dabigatran (a direct thrombin inhibitor), and edoxaban (a factor Xa inhibitor).[2] Other anticoagulants cannot be taken by mouth. These parenteral (non-oral) medicines include low-molecular-weight heparin, fondaparinux, and unfractionated heparin. Some oral medicines are sufficient when taken alone, while others require the use of an additional parenteral blood thinner. Rivaroxaban and apixaban are the typical first-line medicines, and they are sufficient when taken orally.[19] Rivaroxaban is taken once daily, and apixaban is taken twice daily.[5] Warfarin, dabigatran, and edoxaban require the use of a parenteral anticoagulant to initiate oral anticoagulant therapy.[19][125] When warfarin is initiated for VTE treatment, a 5-day minimum of a parenteral anticoagulant[j] together with warfarin is given, which is followed by warfarin-only therapy.[17][18] Warfarin is taken to maintain an international normalized ratio (INR)[k] of 2.0–3.0, with 2.5 as the target.[128] The benefit of taking warfarin declines as the duration of treatment extends,[129] and the risk of bleeding increases with age.[130] Periodic INR monitoring is not necessary when first-line direct oral anticoagulants are used. Overall, anticoagulation therapy is complex, and many circumstances can affect how these therapies are managed.[131]
The duration of anticoagulation therapy (whether it will last 4 to 6 weeks,[5] 6 to 12 weeks, 3 to 6 months,[19] or indefinitely) is a key factor in clinical decision making.[52] When proximal DVT is provoked by surgery or trauma a 3-month course of anticoagulation is standard.[19] When a first VTE is proximal DVT that is either unprovoked or associated with transient non-surgical risk factor, low-dose anticoagulation beyond 3 to 6 months might be used.[19] In those with an annual risk of VTE in excess of 9%, as after an unprovoked episode, extended anticoagulation is a possibility.[132] Those who finish warfarin treatment after idiopathic VTE with an elevated D-dimer level show an increased risk of recurrent VTE (about 9% vs about 4% for normal results), and this result might be used in clinical decision making.[133] Thrombophilia test results rarely play a role in the length of treatment.[80]
Treatment for acute leg DVT is suggested to continue at home for uncomplicated DVT instead of hospitalization. Factors that favor hospitalization include severe symptoms or additional medical issues.[12] Early walking is suggested over bedrest.[134] Graduated compression stockings—which apply higher pressure at the ankles and a lower pressure around the knees[126] can be trialed for symptomatic management of acute DVT symptoms, but they are not recommended for reducing the risk of post-thrombotic syndrome,[125] as the potential benefit of using them for this goal "may be uncertain".[5] Nor are compression stockings likely to reduce VTE recurrence.[135] They are, however, recommended in those with isolated distal DVT.[5]
If someone decides to stop anticoagulation after an unprovoked VTE instead of being on lifelong anticoagulation, aspirin can be used to reduce the risk of recurrence,[136] but it is only about 33% as effective as anticoagulation in preventing recurrent VTE.[52] Statins have also been investigated for their potential to reduce recurrent VTE rates, with some studies suggesting effectiveness.[137]
Investigations for cancer
[edit]An unprovoked VTE might signal the presence of an unknown cancer, as it is an underlying condition in up to 10% of unprovoked cases.[1] A thorough clinical assessment is needed and should include a physical examination, a review of medical history, and universal cancer screening done in people of that age.[19][138] A review of prior imaging is considered worthwhile, as is "reviewing baseline blood test results including full blood count, renal and hepatic function, PT and APTT."[138] It is not recommended practice to obtain tumor markers or a CT of the abdomen and pelvis in asymptomatic individuals.[1] NICE recommends that further investigations are unwarranted in those without relevant signs or symptoms.[138]
Interventions
[edit]Thrombolysis is the injection of an enzyme into the veins to dissolve blood clots, and while this treatment has been proven effective against the life-threatening emergency clots of stroke and heart attacks, randomized controlled trials[139][140][141] have not established a net benefit in those with acute proximal DVT.[5][142] Drawbacks of catheter-directed thrombolysis (the preferred method of administering the clot-busting enzyme[5]) include a risk of bleeding, complexity,[l] and the cost of the procedure.[125] Although, while anticoagulation is the preferred treatment for DVT,[125] thrombolysis is a treatment option for those with the severe DVT form of phlegmasia cerula dorens (bottom left image) and in some younger patients with DVT affecting the iliac and common femoral veins.[12] Of note, a variety of contraindications to thrombolysis exist.[125] In 2020, NICE kept their 2012 recommendations that catheter-directed thrombolysis should be considered in those with iliofemoral DVT who have "symptoms lasting less than 14 days, good functional status, a life expectancy of 1 year or more, and a low risk of bleeding."[138]
A mechanical thrombectomy device can remove DVT clots, particularly in acute iliofemoral DVT (DVT of the major veins in the pelvis), but there is limited data on its efficacy. It is usually combined with thrombolysis, and sometimes temporary IVC filters are placed to protect against PE during the procedure.[143] Catheter-directed thrombolysis with thrombectomy[141] against iliofemoral DVT has been associated with a reduction in the severity of post-thrombotic syndrome at an estimated cost-effectiveness ratio of about $138,000[m] per gained QALY.[144][145] Phlegmasia cerulea dolens might be treated with catheter-directed thrombolysis and/or thrombectomy.[19][143]
In DVT in the arm, the first (topmost) rib can be surgically removed as part of the typical treatment when the DVT is due to thoracic outlet syndrome or Paget–Schroetter syndrome. This treatment involves initial anticoagulation followed by thrombolysis of the subclavian vein and staged first rib resection to relieve the thoracic outlet compression and prevent recurrent DVT.[146]
-
The first rib, which is removed in a first rib resection surgery, is labeled 1 in this image
-
A venogram before catheter-directed thrombolysis for Paget–Schroetter syndrome, a rare and severe arm DVT shown here in a judo practitioner, with highly restricted blood flow shown in the vein
-
After treatment with catheter-directed thrombolysis, blood flow in the axillary and subclavian vein were significantly improved. Afterwards, a first rib resection allowed decompression. This reduces the risk of recurrent DVT and other sequelae from thoracic outlet compression.[147]

The placement of an inferior vena cava filter (IVC filter) is possible when either the standard treatment for acute DVT, anticoagulation, is absolutely contraindicated (not possible), or if someone develops a PE despite being anticoagulated.[138] However, a 2020 NICE review found "little good evidence" for their use.[138] A 2018 study associated IVC filter placement with a 50% reduction in PE, a 70% increase in DVT, and an 18% increase in 30 day mortality when compared to no IVC placement.[1][148] Other studies including a systematic review and meta-analysis did not find a difference in mortality with IVC placement.[30] If someone develops a PE despite being anticoagulated, care should be given to optimize anticoagulation treatment and address other related concerns before considering IVC filter placement.[138]
Field of medicine
[edit]Patients with a history of DVT might be managed by primary care, general internal medicine, hematology, cardiology, vascular surgery, or vascular medicine.[149] Patients suspected of having an acute DVT are often referred to the emergency department for evaluation.[150] Interventional radiology is the specialty that typically places and retrieves IVC filters,[151] and vascular surgery might do catheter directed thrombosis for some severe DVTs.[147]
Prevention
[edit]For the prevention of blood clots in the general population, incorporating leg exercises while sitting down for long periods, or having breaks from a sitting position and walking around, having an active lifestyle, and maintaining a healthy body weight are recommended.[6] Walking increases blood flow through the leg veins.[152] Excess body weight is modifiable unlike most risk factors, and interventions or lifestyle modifications that help someone who is overweight or obese lose weight reduce DVT risk.[88] Avoiding both smoking and a Western pattern diet are thought to reduce risk.[153] Statins have been investigated for primary prevention (prevention of a first VTE), and the JUPITER trial, which used rosuvastatin, has provided some tentative evidence of effectiveness.[14][154] Of the statins, rosuvastatin appears to be the only one with the potential to reduce VTE risk.[155] If so, it appears to reduce risk by about 15%.[153] However, the number needed to treat to prevent one initial VTE is about 2000, limiting its applicability.[156]
Hospital (non-surgical) patients
[edit]Acutely ill hospitalized patients are suggested to receive a parenteral anticoagulant, although the potential net benefit is uncertain.[63] Critically ill hospitalized patients are recommended to either receive unfractionated heparin or low-molecular weight heparin instead of foregoing these medicines.[63]
After surgery
[edit]
Major orthopedic surgery—total hip replacement, total knee replacement, or hip fracture surgery—has a high risk of causing VTE.[157] If prophylaxis is not used after these surgeries, symptomatic VTE has about a 4% chance of developing within 35 days.[158] Following major orthopedic surgery, a blood thinner or aspirin is typically paired with intermittent pneumatic compression, which is the preferred mechanical prophylaxis over graduated compression stockings.[7]
Options for VTE prevention in people following non-orthopedic surgery include early walking, mechanical prophylaxis, and blood thinners (low-molecular-weight heparin and low-dose-unfractionated heparin) depending upon the risk of VTE, risk of major bleeding, and person's preferences.[159] After low-risk surgeries, early and frequent walking is the best preventive measure.[7]
Pregnancy
[edit]The risk of VTE is increased in pregnancy by about four to five times because of a more hypercoagulable state that protects against fatal postpartum hemorrhage.[28] Preventive measures for pregnancy-related VTE were suggested by the American Society of Hematology in 2018.[160] Warfarin, a common vitamin K antagonist, can cause birth defects and is not used for prevention during pregnancy.[161]
Travelers
[edit]
Travelling "is an often cited yet relatively uncommon" cause of VTE.[28] Suggestions for at-risk[n] long-haul travelers include calf exercises, frequent walking, and aisle seating in airplanes to ease walking.[162][163] Graduated compression stockings have sharply reduced the levels of asymptomatic DVT in airline passengers, but the effect on symptomatic DVT, PE, or mortality is unknown, as none of the individuals studied developed these outcomes.[164] However, graduated compression stockings are not suggested for long-haul travelers (>4 hours) without risk factors for VTE. Likewise, neither aspirin nor anticoagulants are suggested in the general population undertaking long-haul travel.[63] Those with significant VTE risk factors[o] undertaking long-haul travel are suggested to use either graduated compression stockings or LMWH for VTE prevention. If neither of these two methods are feasible, then aspirin is suggested.[63]
Prognosis
[edit]DVT is most frequently a disease of older age that occurs in the context of nursing homes, hospitals, and active cancer.[3] It is associated with a 30-day mortality rate of about 6%, with PE being the cause of most of these deaths.[1] Proximal DVT is frequently associated with PE, unlike distal DVT, which is rarely if ever associated with PE.[39] Around 56% of those with proximal DVT also have PE, although a chest CT is not needed simply because of the presence of DVT.[1] If proximal DVT is left untreated, in the following 3 months approximately half of people will experience symptomatic PE.[9]
Another frequent complication of proximal DVT, and the most frequent chronic complication, is post-thrombotic syndrome, where individuals have chronic venous symptoms.[5] Symptoms can include pain, itching, swelling, paresthesia, a sensation of heaviness, and in severe cases, leg ulcers.[5] After proximal DVT, an estimated 20–50% of people develop the syndrome, with 5–10% experiencing severe symptoms.[165] Post-thrombotic syndrome can also be a complication of distal DVT, though to a lesser extent than with proximal DVT.[166]
In the 10 years following an initial VTE, about 30% of people will have a recurrence.[3] VTE recurrence in those with prior DVT is more likely to recur as DVT than PE.[167] Cancer[5] and unprovoked DVT are strong risk factors for recurrence.[60] After initial proximal unprovoked DVT with and without PE, 16–17% of people will have recurrent VTE in the 2 years after they complete their course of anticoagulants. VTE recurrence is less common in distal DVT than proximal DVT.[44][45] In upper extremity DVT, annual VTE recurrence is about 2–4%.[130] After surgery, a provoked proximal DVT or PE has an annual recurrence rate of only 0.7%.[60]
Epidemiology
[edit]About 1.5 out of 1000 adults a year have a first VTE in high-income countries.[168][169] The condition becomes much more common with age.[3] VTE rarely occurs in children, but when it does, it predominantly affects hospitalized children.[170] Children in North America and the Netherlands have VTE rates that range from 0.07 to 0.49 out of 10,000 children annually.[170] Meanwhile, almost 1% of those aged 85 and above experience VTE each year.[3] About 60% of all VTEs occur in those 70 years of age or older.[9] Incidence is about 18% higher in males than in females,[4] though there are ages when VTE is more prevalent in women.[15] VTE occurs in association with hospitalization or nursing home residence about 60% of the time, active cancer about 20% of the time, and a central venous catheter or transvenous pacemaker about 9% of the time.[3]
During pregnancy and after childbirth, acute VTE occurs in about 1.2 of 1000 deliveries. Despite it being relatively rare, it is a leading cause of maternal morbidity and mortality.[160] After surgery with preventive treatment, VTE develops in about 10 of 1000 people after total or partial knee replacement, and in about 5 of 1000 after total or partial hip replacement.[171] About 400,000 Americans develop an initial VTE each year, with 100,000 deaths or more attributable to PE.[169] Asian, Asian-American, Native American, and Hispanic individuals have a lower VTE risk than Whites or Blacks.[4][15] Populations in Asia have VTE rates at 15 to 20% of what is seen in Western countries, with an increase in incidence seen over time.[16] In North American and European populations, around 4–8% of people have a thrombophilia,[89] most commonly factor V leiden and prothrombin G20210A. For populations in China, Japan, and Thailand, deficiences in protein S, protein C, and antithrombin predominate.[172] Non-O blood type is present in around 50% of the general population and varies with ethnicity, and it is present in about 70% of those with VTE.[90][173]
DVT occurs in the upper extremities in about 4–10% of cases,[11] with an incidence of 0.4–1.0 people out of 10,000 a year.[5] A minority of upper extremity DVTs are due to Paget–Schroetter syndrome, also called effort thrombosis, which occurs in 1–2 people out of 100,000 a year, usually in athletic males around 30 years of age or in those who do significant amounts of overhead manual labor.[69][147]
Social
[edit]
Being on blood thinners because of DVT can be life-changing because it can prevent lifestyle activities such as contact or winter sports to prevent bleeding after potential injuries.[175] Head injuries prompting brain bleeds are of particular concern. This has caused NASCAR driver Brian Vickers to forego participation in races. Professional basketball players including NBA players Chris Bosh and hall of famer Hakeem Olajuwon have dealt with recurrent blood clots,[176] and Bosh's career was significantly hampered by DVT and PE.[177]
Tennis star Serena Williams was hospitalized in 2011 for PE thought to have originated from DVT.[178] Years later, in 2017, due to her knowledge of DVT and PE, Serena accurately advocated for herself to have a PE diagnosed and treated. During this encounter with VTE, she was hospitalized after a C-section surgery and was off of blood thinners. After feeling the sudden onset of a PE symptom, shortness of breath, she told her nurse and requested a CT scan and an IV heparin drip, all while gasping for air. She started to receive an ultrasound to look for DVT in the legs, prompting her to express dissatisfaction to the medical staff that they were not looking for clots where she had symptoms (her lungs), and they were not yet treating her presumed PE. After being diagnosed with PE and not DVT, and after receiving heparin by IV, the coughing from the PE caused her C-section surgical site to open and the heparin contributed to bleeding at the site. Serena later received an IVC filter while in the hospital.[174][179]
Other notable people have been affected by DVT. Former United States President Richard Nixon had recurrent DVT,[180] and so has former Secretary of State Hillary Clinton. She was first diagnosed while First Lady in 1998 and again in 2009.[181] Dick Cheney was diagnosed with an episode while Vice President,[182] and TV show host Regis Philbin had DVT after hip-replacement surgery.[183] DVT has also contributed to the deaths of famous people. For example, DVT and PE played a role in rapper Heavy D's death at age 44.[184] NBC journalist David Bloom died at age 39 while covering the Iraq War from a PE that was thought to have progressed from a missed DVT,[185] and actor Jimmy Stewart had DVT that progressed to a PE when he was 89.[183][186]
History
[edit]
The book Sushruta Samhita, an Ayurvedic text published around 600–900 BC, contains what has been cited as the first description of DVT.[187] In 1271, DVT symptoms in the leg of a 20-year-old male were described in a French manuscript, which has been cited as the first case or the first Western reference to DVT.[187][188]
In 1856, German physician and pathologist Rudolf Virchow published his analysis after the insertion of foreign bodies into the jugular veins of dogs, which migrated to the pulmonary arteries. These foreign bodies caused pulmonary emboli, and Virchow was focused on explaining their consequences.[189] He cited three factors, which are now understood as hypercoaguability, stasis, and endothelial injury.[190] It was not until 1950 that this framework was cited as Virchow's triad,[189] but the teaching of Virchow's triad has continued in light of its utility as a theoretical framework and as a recognition of the significant progress Virchow made in expanding the understanding of VTE.[189][190]
Methods to observe DVT by ultrasound were established in the 1960s.[120] Diagnoses were commonly performed by impedance plethysmography in the 1970s and 1980s,[191] but ultrasound, particularly after utility of probe compression was demonstrated in 1986, became the preferred diagnostic method.[187] Yet, in the mid-1990s, contrast venography and impedance plethysmography were still described as common.[192]
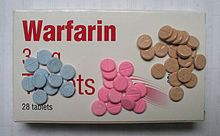
Multiple pharmacological therapies for DVT were introduced in the 20th century: oral anticoagulants in the 1940s, subcutaneous injections of LDUH in 1962 and subcutaneous injections of LMWH in 1982.[193] 1974 was when vascular inflammation and venous thrombosis were first proposed to be interrelated.[112] For around 50 years, a months-long warfarin (Coumadin) regimen was the mainstay of pharmacological treatment.[194][195] To avoid the blood monitoring required with warfarin and the injections required by heparin and heparin-like medicines, direct oral anticoagulants (DOACs) were developed.[195] In the late 2000s to early 2010s, DOACs—including rivaroxaban (Xarelto), apixaban (Eliquis), and dabigatran (Pradaxa)—came to the market.[60] The New York Times described a "furious battle" among the three makers of these drugs "for the prescription pads of doctors".[194]
Economics
[edit]VTE costs the US healthcare system about $7 to 10 billion dollars annually.[169] Initial and average DVT costs for a hospitalized US patient is about $10,000 (2015 estimate).[196] In Europe, the costs for an initial VTE hospitalization are significantly less, costing about €2000 to 4000 (2011 estimate).[197] Post-thrombotic syndrome is a significant contributor to DVT follow-up costs.[198] Outpatient treatment significantly reduces costs, and treatment costs for PE exceed those of DVT.[199]
Research directions
[edit]A 2019 study published in Nature Genetics reported more than doubling the known genetic loci associated with VTE.[14] In their updated 2018 clinical practice guidelines, the American Society of Hematology identified 29 separate research priorities, most of which related to patients who are acutely or critically ill.[63] Inhibition of factor XI, P-selectin, E-selectin, and a reduction in formation of neutrophil extracellular traps are potential therapies that might treat VTE without increasing bleeding risk.[200]
Notes
[edit]- ^ Venous thrombosis associated with drainage from the brain (cerebral venous sinus thrombosis), eyes (retinal vein thrombosis), spleen and intestines (splanchnic vein thrombosis), liver (Budd–Chiari syndrome), kidneys (renal vein thrombosis), and ovaries (ovarian vein thrombosis) are more unusual forms of venous thrombosis and they are considered as separate diseases.[10]
- ^ Third-generation combined oral contraceptives (COCs) have an approximate two to three times higher risk than second-generation COCs.[64] Progestogen-only pill use is not associated with increased VTE risk.[87]
- ^ Type I[58]
- ^ "It is important to note that smoking is not an independent risk factor, although it increases the risk for cancers and other comorbidities and works synergistically with other independent risk factors."[98]
- ^ The term 'thrombophilia' as used here applies to the five inherited abnormalities of antithrombin, protein C, protein S, factor V, and prothrombin, as is done elsewhere.[89][99] These 5 genetic factors have been referred to as the classical thrombophilias.[100]
- ^ An elevated level is greater than 250 ng/mL D-dimer units (DDU) or greater than 0.5 μg/mL fibrinogen equivalent units (FEU). A normal level is below these values.[113]
- ^ The Wells score as displayed here is the more recent modified score, which added a criterion for a previous documented DVT and increased the time range after surgery to 12 weeks from 4 weeks.[117]
- ^ Subsartorial is a proposed name for a section of the femoral vein.[121]
- ^ Evidence for anticoagulation comes from studies other than definitive randomized controlled trials that demonstrate efficacy and safety for anticoagulation vs. placebo or using NSAIDs.[123]
- ^ The international normalized ratio should be ≥ 2.0 for 24 hours minimum,[18] but if the ratio is > 3.0, then the parenteral anticoagulant is not needed for five days.[126]
- ^ An INR is determined from the ratio of a patient's prothrombin time (PT) to a standardized control PT. A normal INR for those not on anticoagulation is 1.0. A value of 5.0 or higher is considered a critical finding because of an increased risk of bleeding.[127]
- ^ "Up to 83% of patients treated by any catheter-based therapy, need adjunctive angioplasty, and stenting".[5]
- ^ Estimated in United States dollars, estimate published in 2019
- ^ Including those with "previous VTE, recent surgery or trauma, active malignancy, pregnancy, estrogen use, advanced age, limited mobility, severe obesity, or known thrombophilic disorder"[162]
- ^ For example "recent surgery, history of VTE, postpartum women, active malignancy, or ≥2 risk factors, including combinations of the above with hormone replacement therapy, obesity, or pregnancy"[63]
References
[edit]- ^ a b c d e f g h i j k l m Kruger PC, Eikelboom JW, Douketis JD, Hankey GJ (June 2019). "Deep vein thrombosis: update on diagnosis and management". The Medical Journal of Australia. 210 (11): 516–24. doi:10.5694/mja2.50201. PMID 31155730. S2CID 173995098.
- ^ a b c d e f Bartholomew JR (December 2017). "Update on the management of venous thromboembolism". Cleveland Clinic Journal of Medicine. 84 (12 Suppl 3): 39–46. doi:10.3949/ccjm.84.s3.04. PMID 29257737. S2CID 3707226.
- ^ a b c d e f g h i Heit JA, Spencer FA, White RH (January 2016). "The epidemiology of venous thromboembolism". Journal of Thrombosis and Thrombolysis. 41 (1): 3–14. doi:10.1007/s11239-015-1311-6. PMC 4715842. PMID 26780736.
- ^ a b c d e f g Crous-Bou M, Harrington LB, Kabrhel C (November 2016). "Environmental and genetic risk factors associated with venous thromboembolism". Seminars in Thrombosis and Hemostasis. 42 (8): 808–20. doi:10.1055/s-0036-1592333. PMC 5146955. PMID 27764878.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y Mazzolai L, Aboyans V, Ageno W, Agnelli G, Alatri A, Bauersachs R, et al. (December 2018). "Diagnosis and management of acute deep vein thrombosis: a joint consensus document from the European Society of Cardiology working groups of aorta and peripheral vascular diseases and pulmonary circulation and right ventricular function". European Heart Journal. 39 (47): 4208–18. doi:10.1093/eurheartj/ehx003. PMID 28329262.
- ^ a b "What is Venous Thromboembolism?". Centers for Disease Control and Prevention. 14 March 2019. Retrieved 6 January 2020.
- ^ a b c d Anderson DR, Morgano GP, Bennett C, Dentali F, Francis CW, Garcia DA, et al. (December 2019). "American Society of Hematology 2019 guidelines for management of venous thromboembolism: prevention of venous thromboembolism in surgical hospitalized patients". Blood Advances. 3 (23): 3898–944. doi:10.1182/bloodadvances.2019000975. PMC 6963238. PMID 31794602.
- ^ Raskob GE, Angchaisuksiri P, Blanco AN, Buller H, Gallus A, Hunt BJ, et al. (November 2014). "Thrombosis: a major contributor to global disease burden". Arteriosclerosis, Thrombosis, and Vascular Biology. 34 (11): 2363–71. doi:10.1161/ATVBAHA.114.304488. PMID 25304324.
- ^ a b c d e Phillippe HM (December 2017). "Overview of venous thromboembolism". The American Journal of Managed Care. 23 (20 Suppl): S376–82. PMID 29297660. Archived from the original on 30 January 2020. Retrieved 30 January 2020.
- ^ a b Abbattista M, Capecchi M, Martinelli I (January 2020). "Treatment of unusual thrombotic manifestations". Blood. 135 (5): 326–34. doi:10.1182/blood.2019000918. PMID 31917405.
- ^ a b c d e f g Heil J, Miesbach W, Vogl T, Bechstein WO, Reinisch A (April 2017). "Deep vein thrombosis of the upper extremity". Deutsches Ärzteblatt International. 114 (14): 244–49. doi:10.3238/arztebl.2017.0244. PMC 5415909. PMID 28446351.
- ^ a b c d e Ortel TL, Neumann I, Ageno W, Beyth R, Clark NP, Cuker A, et al. (October 2020). "American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism". Blood Advances. 4 (19): 4693–738. doi:10.1182/bloodadvances.2020001830. PMC 7556153. PMID 33007077.
- ^ a b Borgel D, Bianchini E, Lasne D, Pascreau T, Saller F (December 2019). "Inflammation in deep vein thrombosis: a therapeutic target?". Hematology. 24 (1): 742–50. doi:10.1080/16078454.2019.1687144. PMID 31736432.
- ^ a b c d e f g Klarin D, Busenkell E, Judy R, Lynch J, Levin M, Haessler J, et al. (November 2019). "Genome-wide association analysis of venous thromboembolism identifies new risk loci and genetic overlap with arterial vascular disease". Nature Genetics. 51 (11): 1574–79. doi:10.1038/s41588-019-0519-3. PMC 6858581. PMID 31676865.
- ^ a b c Wendelboe AM, Raskob GE (April 2016). "Global burden of thrombosis: epidemiologic aspects". Circulation Research. 118 (9): 1340–47. doi:10.1161/CIRCRESAHA.115.306841. PMID 27126645.
- ^ a b Lee LH, Gallus A, Jindal R, Wang C, Wu CC (December 2017). "Incidence of venous thromboembolism in Asian populations: a systematic review". Thrombosis and Haemostasis. 117 (12): 2243–60. doi:10.1160/TH17-02-0134. PMID 29212112.
- ^ a b Keeling D, Alikhan R (June 2013). "Management of venous thromboembolism – controversies and the future". British Journal of Haematology. 161 (6): 755–63. doi:10.1111/bjh.12306. PMID 23531017.
- ^ a b c Guyatt et al. 2012, p. 20S: 2.4.
- ^ a b c d e f g h i j Tran HA, Gibbs H, Merriman E, Curnow JL, Young L, Bennett A, et al. (March 2019). "New guidelines from the Thrombosis and Haemostasis Society of Australia and New Zealand for the diagnosis and management of venous thromboembolism". The Medical Journal of Australia. 210 (5): 227–35. doi:10.5694/mja2.50004. hdl:11343/285435. PMID 30739331. S2CID 73433650.
- ^ a b c d Ratchford EV, Evans NS (March 2017). "Approach to lower extremity edema". Current Treatment Options in Cardiovascular Medicine. 19 (3): 16. doi:10.1007/s11936-017-0518-6. PMID 28290004. S2CID 34922038.
- ^ a b c Moll S (March 2008). "A clinical perspective of venous thromboembolism". Arteriosclerosis, Thrombosis, and Vascular Biology. 28 (3): 373–79. doi:10.1161/ATVBAHA.108.162818. PMID 18296592.
- ^ a b Lloyd NS, Douketis JD, Moinuddin I, Lim W, Crowther MA (March 2008). "Anticoagulant prophylaxis to prevent asymptomatic deep vein thrombosis in hospitalized medical patients: a systematic review and meta-analysis". Journal of Thrombosis and Haemostasis. 6 (3): 405–14. doi:10.1111/j.1538-7836.2007.02847.x. PMID 18031292.
- ^ Conklin P, Soares GM, Dubel GJ, Ahn SH, Murphy TP (December 2009). "Acute deep vein thrombosis (DVT): evolving treatment strategies and endovascular therapy" (PDF). Medicine and Health, Rhode Island. 92 (12): 394–97. PMID 20066826. Archived (PDF) from the original on 6 February 2013.
- ^ Stubbs MJ, Mouyis M, Thomas M (February 2018). "Deep vein thrombosis". BMJ. 360 (8142): k351. doi:10.1136/bmj.k351. PMID 29472180. S2CID 3454404.
- ^ Casella IB, Bosch MA, Sabbag CR (2009). "Incidence and risk factors for bilateral deep venous thrombosis of the lower limbs". Angiology. 60 (1): 99–103. doi:10.1177/0003319708316897. PMID 18504268. S2CID 30043830.
- ^ Kennedy D, Setnik G, Li J (November 2001). "Physical examination findings in deep venous thrombosis". Emergency Medicine Clinics of North America. 19 (4): 869–76. doi:10.1016/s0733-8627(05)70223-6. PMID 11762276.
- ^ Campello E, Ilich A, Simioni P, Key NS (August 2019). "The relationship between pancreatic cancer and hypercoagulability: a comprehensive review on epidemiological and biological issues". British Journal of Cancer. 121 (5): 359–71. doi:10.1038/s41416-019-0510-x. PMC 6738049. PMID 31327867.
- ^ a b c Turetz M, Sideris AT, Friedman OA, Triphathi N, Horowitz JM (June 2018). "Epidemiology, pathophysiology, and natural history of pulmonary embolism". Seminars in Interventional Radiology. 35 (2): 92–98. doi:10.1055/s-0038-1642036. PMC 5986574. PMID 29872243.
- ^ a b Doherty S (November 2017). "Pulmonary embolism: an update". Australian Family Physician. 46 (11): 816–20. PMID 29101916.
- ^ a b c Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, et al. (January 2020). "2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS)". European Heart Journal. 41 (4): 543–603. doi:10.1093/eurheartj/ehz405. PMID 31504429.
- ^ Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ (January 2011). "Trends in management of phlegmasia cerulea dolens". Vascular and Endovascular Surgery. 45 (1): 5–14. doi:10.1177/1538574410388309. PMID 21193462. S2CID 64951.
- ^ Turner DPB (November 1952). "A case of phlegmasia cerulea dolens". British Medical Journal. 2 (4795): 1183–85. doi:10.1136/bmj.2.4795.1183. PMC 2021962. PMID 12997687.
- ^ Aggarwal DG, Bhojraj SS, Behrainwalla AA, Jani CK, Mehta SS (January 2018). "Phlegmasia cerulea dolens following heparin-induced thrombocytopenia". Indian Journal of Critical Care Medicine. 22 (1): 51–52. doi:10.4103/ijccm.IJCCM_183_16. PMC 5793026. PMID 29422736.
- ^ Owings JT (2005). "Management of venous thromboembolism". ACS Surgery. American College of Surgeons. Archived from the original on 27 January 2012. Retrieved 16 January 2012.
- ^ Mazer BA, Hughes PG (November 2018). "Pacemaker-associated phlegmasia cerulea dolens treated with catheter-directed thrombolysis". Clinical Practice and Cases in Emergency Medicine. 2 (4): 316–19. doi:10.5811/cpcem.2018.8.39444. PMC 6230348. PMID 30443615.
- ^ Abdul W, Hickey B, Wilson C (April 2016). "Lower extremity compartment syndrome in the setting of iliofemoral deep vein thrombosis, phlegmasia cerulea dolens and factor VII deficiency". BMJ Case Reports. 2016: bcr2016215078. doi:10.1136/bcr-2016-215078. PMC 4854131. PMID 27113791.
- ^ Zietz A, Sutter R, De Marchis GM (2020). "Deep vein thrombosis and pulmonary embolism among patients with a cryptogenic stroke linked to patent foramen ovale – a review of the literature". Frontiers in Neurology. 11: 336. doi:10.3389/fneur.2020.00336. PMC 7214694. PMID 32431661.
- ^ Pristipino C, Sievert H, D'Ascenzo F, Mas JL, Meier B, Scacciatella P, et al. (January 2019). "European position paper on the management of patients with patent foramen ovale. General approach and left circulation thromboembolism". EuroIntervention. 14 (13): 1389–402. doi:10.4244/EIJ-D-18-00622. hdl:2318/1691212. PMID 30141306.
- ^ a b c d e f g Bates SM, Jaeschke R, Stevens SM, Goodacre S, Wells PS, Stevenson MD, et al. (February 2012). "Diagnosis of DVT: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines". Chest. 141 (2 Suppl): e351S–e418S. doi:10.1378/chest.11-2299. PMC 3278048. PMID 22315267.
- ^ Arumilli BR, Lenin Babu V, Paul AS (January 2008). "Painful swollen leg – think beyond deep vein thrombosis or Baker's cyst". World Journal of Surgical Oncology. 6: 6. doi:10.1186/1477-7819-6-6. PMC 2244628. PMID 18205917.
- ^ Bauersachs RM (September 2012). "Clinical presentation of deep vein thrombosis and pulmonary embolism". Best Practice & Research Clinical Haematology. 25 (3): 243–51. doi:10.1016/j.beha.2012.07.004. PMID 22959541.
- ^ a b c Mukhopadhyay S, Johnson TA, Duru N, Buzza MS, Pawar NR, Sarkar R, et al. (2019). "Fibrinolysis and inflammation in venous thrombus resolution". Frontiers in Immunology. 10: 1348. doi:10.3389/fimmu.2019.01348. PMC 6587539. PMID 31258531.
- ^ Karande GY, Hedgire SS, Sanchez Y, Baliyan V, Mishra V, Ganguli S, et al. (December 2016). "Advanced imaging in acute and chronic deep vein thrombosis". Cardiovascular Diagnosis and Therapy. 6 (6): 493–507. doi:10.21037/cdt.2016.12.06. PMC 5220209. PMID 28123971.
- ^ a b Khan F, Rahman A, Carrier M, Kearon C, Weitz JI, Schulman S, et al. (July 2019). "Long term risk of symptomatic recurrent venous thromboembolism after discontinuation of anticoagulant treatment for first unprovoked venous thromboembolism event: systematic review and meta-analysis". BMJ. 366 (8209): l4363. doi:10.1136/bmj.l4363. PMC 6651066. PMID 31340984.
- ^ a b "Significant risk of another thrombosis remains if anticoagulation is stopped". NIHR Evidence (Plain English summary). 31 October 2019. doi:10.3310/signal-000830. S2CID 242392407.
- ^ Casella IB, Bosch MA, Sabbag CR (2009). "Incidence and risk factors for bilateral deep venous thrombosis of the lower limbs". Angiology. 60 (1): 99–103. doi:10.1177/0003319708316897. PMID 18504268. S2CID 30043830.
- ^ Johnson SA, Stevens SM, Woller SC, Lake E, Donadini M, Cheng J, et al. (February 2010). "Risk of deep vein thrombosis following a single negative whole-leg compression ultrasound: a systematic review and meta-analysis". JAMA. 303 (5): 438–45. doi:10.1001/jama.2010.43. PMID 20124539.
- ^ Scarvelis D, Wells PS (October 2006). "Diagnosis and treatment of deep-vein thrombosis". Canadian Medical Association Journal. 175 (9): 1087–92. doi:10.1503/cmaj.060366. PMC 1609160. PMID 17060659.
Scarvelis D, Wells PS (November 2007). "Correction: Diagnosis and treatment of deep-vein thrombosis". Canadian Medical Association Journal. 177 (11): 1392. doi:10.1503/cmaj.071550. PMC 2072980. - ^ Galanaud JP, Bosson JL, Quéré I (September 2011). "Risk factors and early outcomes of patients with symptomatic distal vs. proximal deep-vein thrombosis". Current Opinion in Pulmonary Medicine. 17 (5): 387–91. doi:10.1097/MCP.0b013e328349a9e3. PMID 21832920. S2CID 33536953.
- ^ Utter GH, Dhillon TS, Salcedo ES, Shouldice DJ, Reynolds CL, Humphries MD, et al. (September 2016). "Therapeutic anticoagulation for isolated calf deep vein thrombosis". JAMA Surgery. 151 (9): e161770. doi:10.1001/jamasurg.2016.1770. PMID 27437827.
- ^ Comerota AJ, Kearon C, Gu CS, Julian JA, Goldhaber SZ, Kahn SR, et al. (February 2019). "Endovascular Thrombus Removal for Acute Iliofemoral Deep Vein Thrombosis". Circulation. 139 (9): 1162–73. doi:10.1161/CIRCULATIONAHA.118.037425. PMC 6389417. PMID 30586751.
- ^ a b c d Kearon C, Kahn SR (January 2020). "Long-term treatment of venous thromboembolism". Blood. 135 (5): 317–25. doi:10.1182/blood.2019002364. PMID 31917402.
- ^ Ageno W, Farjat A, Haas S, Weitz JI, Goldhaber SZ, Turpie AGG, et al. (February 2021). "Provoked versus unprovoked venous thromboembolism: Findings from GARFIELD-VTE". Research and Practice in Thrombosis and Haemostasis. 5 (2): 326–41. doi:10.1002/rth2.12482. PMC 7938631. PMID 33733032.
- ^ Piazza G (19 October 2019). "Clot Chronicles: unprovoked vs. provoked VTE". North American Thrombosis Forum. Archived from the original on 8 May 2021. Retrieved 8 May 2021.
- ^ a b c d e Najem MY, Couturaud F, Lemarié CA (May 2020). "Cytokine and chemokine regulation of venous thromboembolism". Journal of Thrombosis and Haemostasis. 18 (5): 1009–19. doi:10.1111/jth.14759. PMID 32020753. S2CID 211037046.
- ^ a b c d e f Reitsma PH, Versteeg HH, Middeldorp S (March 2012). "Mechanistic view of risk factors for venous thromboembolism". Arteriosclerosis, Thrombosis, and Vascular Biology. 32 (3): 563–68. doi:10.1161/ATVBAHA.111.242818. PMID 22345594.
- ^ Kujovich JL (January 2011). "Factor V Leiden thrombophilia". Genetics in Medicine. 13 (1): 1–16. doi:10.1097/GIM.0b013e3181faa0f2. PMID 21116184.
- ^ a b c d e Lijfering WM, Rosendaal FR, Cannegieter SC (June 2010). "Risk factors for venous thrombosis – current understanding from an epidemiological point of view". British Journal of Haematology. 149 (6): 824–33. doi:10.1111/j.1365-2141.2010.08206.x. PMID 20456358.
- ^ Tzoran I, Hoffman R, Monreal M (October 2018). "Hemostasis and thrombosis in the oldest old". Seminars in Thrombosis and Hemostasis. 44 (7): 624–31. doi:10.1055/s-0038-1657779. PMID 29920621. S2CID 49313388.
- ^ a b c d Keeling D, Alikhan R (June 2013). "Management of venous thromboembolism – controversies and the future". British Journal of Haematology. 161 (6): 755–63. doi:10.1111/bjh.12306. PMID 23531017.
- ^ Previtali E, Bucciarelli P, Passamonti SM, Martinelli I (April 2011). "Risk factors for venous and arterial thrombosis". Blood Transfusion. 9 (2): 120–38. doi:10.2450/2010.0066-10. PMC 3096855. PMID 21084000.
- ^ a b c d e f g h i Martinelli I, Bucciarelli P, Mannucci PM (February 2010). "Thrombotic risk factors: basic pathophysiology". Critical Care Medicine. 38 (2 Suppl): S3-9. doi:10.1097/CCM.0b013e3181c9cbd9. PMID 20083911. S2CID 34486553.
- ^ a b c d e f g Schünemann HJ, Cushman M, Burnett AE, Kahn SR, Beyer-Westendorf J, Spencer FA, et al. (November 2018). "American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients". Blood Advances. 2 (22): 3198–225. doi:10.1182/bloodadvances.2018022954. PMC 6258910. PMID 30482763.
- ^ a b Wong P, Baglin T (2012). "Epidemiology, risk factors and sequelae of venous thromboembolism". Phlebology. 27 (Suppl 2): 2–11. doi:10.1258/phleb.2012.012S31. PMID 22457300. S2CID 13564168.
- ^ a b Rosendaal FR, Reitsma PH (July 2009). "Genetics of venous thrombosis". Journal of Thrombosis and Haemostasis. 7 (Suppl 1): 301–04. doi:10.1111/j.1538-7836.2009.03394.x. PMID 19630821.
- ^ Ishida T, Sakurai H, Watanabe K, Iwashita S, Mimura M, Uchida H (July 2016). "Incidence of deep vein thrombosis in catatonic patients: A chart review". Psychiatry Research. 241: 61–5. doi:10.1016/j.psychres.2016.04.105. PMID 27156025. S2CID 207452463.
- ^ Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. (June 2016). "Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association". Stroke. 47 (6): e98–e169. doi:10.1161/STR.0000000000000098. PMID 27145936.
- ^ Béliard S, Feuvrier D, Ducroux E, Salomon du Mont L (2018). "May Thurner syndrome revealed by left calf venous claudication during running, a case report". BMC Sports Science, Medicine & Rehabilitation. 10: 3. doi:10.1186/s13102-018-0092-6. PMC 5796503. PMID 29435334.
- ^ a b Hangge P, Rotellini-Coltvet L, Deipolyi AR, Albadawi H, Oklu R (December 2017). "Paget-Schroetter syndrome: treatment of venous thrombosis and outcomes". Cardiovascular Diagnosis and Therapy. 7 (Suppl 3): S285–90. doi:10.21037/cdt.2017.08.15. PMC 5778512. PMID 29399532.
- ^ Jabri H, Mukherjee S, Sanghavi D, Chalise S (2014). "Bilateral upper extremity DVT in a 43-year-old man: is it thoracic outlet syndrome?!". Case Reports in Medicine. 2014: 758010. doi:10.1155/2014/758010. PMC 4129160. PMID 25140182.
- ^ Beristain-Covarrubias N, Perez-Toledo M, Thomas MR, Henderson IR, Watson SP, Cunningham AF (2019). "Understanding infection-induced thrombosis: lessons learned from animal models". Frontiers in Immunology. 10: 2569. doi:10.3389/fimmu.2019.02569. PMC 6848062. PMID 31749809.
- ^ Kaplan D, Casper TC, Elliott CG, Men S, Pendleton RC, Kraiss LW, et al. (November 2015). "VTE incidence and risk factors in patients with severe sepsis and septic shock". Chest. 148 (5): 1224–30. doi:10.1378/chest.15-0287. PMC 4631038. PMID 26111103.
- ^ Jiménez D, García-Sanchez A, Rali P, Muriel A, Bikdeli B, Ruiz-Artacho P, et al. (March 2021). "Incidence of VTE and bleeding among hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis". Chest. 159 (3): 1182–96. doi:10.1016/j.chest.2020.11.005. PMC 7670889. PMID 33217420.
- ^ Rokx C, Borjas Howard JF, Smit C, Wit FW, Pieterman ED, Reiss P, et al. (May 2020). "Risk of recurrent venous thromboembolism in patients with HIV infection: A nationwide cohort study". PLOS Medicine. 17 (5): e1003101. doi:10.1371/journal.pmed.1003101. PMC 7224453. PMID 32407386.
- ^ Danwang C, Bigna JJ, Awana AP, Nzalie RN, Robert A (February 2021). "Global epidemiology of venous thromboembolism in people with active tuberculosis: a systematic review and meta-analysis". Journal of Thrombosis and Thrombolysis. 51 (2): 502–12. doi:10.1007/s11239-020-02211-7. PMID 32627124. S2CID 220337035.
- ^ Borjas-Howard JF, Leeuw K, Rutgers A, Meijer K, Tichelaar VYIG (March 2019). "Risk of recurrent venous thromboembolism in autoimmune diseases: a systematic review of the literature" (PDF). Seminars in Thrombosis and Hemostasis. 45 (2): 141–49. doi:10.1055/s-0038-1661387. PMID 29954011. S2CID 49606106.
- ^ a b c Branchford BR, Carpenter SL (2018). "The role of inflammation in venous thromboembolism". Frontiers in Pediatrics. 6: 142. doi:10.3389/fped.2018.00142. PMC 5974100. PMID 29876337.
- ^ Henke PK, Kahn SR, Pannucci CJ, Secemksy EA, Evans NS, Khorana AA, et al. (June 2020). "Call to action to prevent venous thromboembolism in hospitalized patients: a policy statement from the American Heart Association". Circulation. 141 (24): e914–31. doi:10.1161/CIR.0000000000000769. PMID 32375490.
- ^ Becatti M, Emmi G, Bettiol A, Silvestri E, Di Scala G, Taddei N, Prisco D, Fiorillo C (March 2019). "Behçet's syndrome as a tool to dissect the mechanisms of thrombo-inflammation: clinical and pathogenetic aspects". Clinical and Experimental Immunology. 195 (3): 322–33. doi:10.1111/cei.13243. PMC 6378375. PMID 30472725.
- ^ a b Baglin T (April 2012). "Inherited and acquired risk factors for venous thromboembolism". Seminars in Respiratory and Critical Care Medicine. 33 (2): 127–37. doi:10.1055/s-0032-1311791. PMID 22648484. S2CID 6925903.
- ^ Knight CL, Nelson-Piercy C (2017). "Management of systemic lupus erythematosus during pregnancy: challenges and solutions". Open Access Rheumatology: Research and Reviews. 9: 37–53. doi:10.2147/OARRR.S87828. PMC 5354538. PMID 28331377.
- ^ Svenungsson E, Antovic A (January 2020). "The antiphospholipid syndrome – often overlooked cause of vascular occlusions?". Journal of Internal Medicine. 287 (4): 349–72. doi:10.1111/joim.13022. PMID 31957081.
- ^ Falanga A, Russo L, Milesi V, Vignoli A (October 2017). "Mechanisms and risk factors of thrombosis in cancer". Critical Reviews in Oncology/Hematology. 118: 79–83. doi:10.1016/j.critrevonc.2017.08.003. PMID 28917273.
- ^ a b c Fernandes CJ, Morinaga LTK, Alves JL, Castro MA, Calderaro D, Jardim CVP, et al. (March 2019). "Cancer-associated thrombosis: the when, how and why". European Respiratory Review. 28 (151): 180119. doi:10.1183/16000617.0119-2018. PMC 9488553. PMID 30918022.
- ^ Levi M, Scully M (February 2018). "How I treat disseminated intravascular coagulation". Blood. 131 (8): 845–54. doi:10.1182/blood-2017-10-804096. PMID 29255070.
- ^ a b c d Bovill EG, van der Vliet A (2011). "Venous valvular stasis-associated hypoxia and thrombosis: what is the link?". Annual Review of Physiology. 73: 527–45. doi:10.1146/annurev-physiol-012110-142305. PMID 21034220.
- ^ Mantha S, Karp R, Raghavan V, Terrin N, Bauer KA, Zwicker JI (August 2012). "Assessing the risk of venous thromboembolic events in women taking progestin-only contraception: a meta-analysis". BMJ. 345 (7872): e4944. doi:10.1136/bmj.e4944. PMC 3413580. PMID 22872710.
- ^ a b c d Shaheen K, Alraies MC, Alraiyes AH, Christie R (April 2012). "Factor V Leiden: how great is the risk of venous thromboembolism?". Cleveland Clinic Journal of Medicine. 79 (4): 265–72. doi:10.3949/ccjm.79a.11072. PMID 22473726. S2CID 23139811.
- ^ a b c Varga EA, Kujovich JL (January 2012). "Management of inherited thrombophilia: guide for genetics professionals". Clinical Genetics. 81 (1): 7–17. doi:10.1111/j.1399-0004.2011.01746.x. PMID 21707594. S2CID 9305488.
- ^ a b c Dentali F, Sironi AP, Ageno W, Turato S, Bonfanti C, Frattini F, et al. (July 2012). "Non-O blood type is the commonest genetic risk factor for VTE: results from a meta-analysis of the literature". Seminars in Thrombosis and Hemostasis. 38 (5): 535–48. doi:10.1055/s-0032-1315758. PMID 22740183. S2CID 5203474.
- ^ Paulsen B, Skille H, Smith EN, Hveem K, Gabrielsen ME, Brækkan SK, et al. (October 2019). "Fibrinogen gamma gene rs2066865 and risk of cancer-related venous thromboembolism". Haematologica. 105 (7): 1963–68. doi:10.3324/haematol.2019.224279. PMC 7327659. PMID 31582554.
- ^ Eslamiyeh H, Ashrafzadeh F, Akhondian J, Beiraghi Toosi M (2015). "Homocystinuria: a rare disorder presenting as cerebral sinovenous thrombosis". Iranian Journal of Child Neurology. 9 (2): 53–57. PMC 4515342. PMID 26221164.
- ^ Ortel TL, Erkan D, Kitchens CS (September 2015). "How I treat catastrophic thrombotic syndromes". Blood. 126 (11): 1285–93. doi:10.1182/blood-2014-09-551978. PMID 26179082.
- ^ Lazo-Langner A, Kovacs MJ, Hedley B, Al-Ani F, Keeney M, Louzada ML, et al. (June 2015). "Screening of patients with idiopathic venous thromboembolism for paroxysmal nocturnal hemoglobinuria clones". Thrombosis Research. 135 (6): 1107–09. doi:10.1016/j.thromres.2015.04.006. PMID 25890452.
- ^ Lu HY, Liao KM (August 2018). "Increased risk of deep vein thrombosis in end-stage renal disease patients". BMC Nephrology. 19 (1): 204. doi:10.1186/s12882-018-0989-z. PMC 6097196. PMID 30115029.
- ^ Reikvam H, Tiu RV (April 2012). "Venous thromboembolism in patients with essential thrombocythemia and polycythemia vera". Leukemia. 26 (4): 563–71. doi:10.1038/leu.2011.314. PMID 22076463.
- ^ Kyrle PA, Eichinger S (2005). "Deep vein thrombosis". Lancet. 365 (9465): 1163–74. doi:10.1016/S0140-6736(05)71880-8. PMID 15794972. S2CID 54379879.
- ^ McLendon K, Goyal A, Bansal P, Attia M (2020). "Deep venous thrombosis risk factors". StatPearls [Internet]. Treasure Island, FL. PMID 29262230.
- ^ Middeldorp S (2011). "Is thrombophilia testing useful?". Hematology. American Society of Hematology. Education Program. 2011 (1): 150–55. doi:10.1182/asheducation-2011.1.150. PMID 22160027.
- ^ Zöller B, Svensson PJ, Dahlbäck B, Lind-Hallden C, Hallden C, Elf J (September 2020). "Genetic risk factors for venous thromboembolism". Expert Review of Hematology. 13 (9): 971–81. doi:10.1080/17474086.2020.1804354. PMID 32731838.
- ^ Palareti G, Schellong S (January 2012). "Isolated distal deep vein thrombosis: what we know and what we are doing". Journal of Thrombosis and Haemostasis. 10 (1): 11–19. doi:10.1111/j.1538-7836.2011.04564.x. PMID 22082302.
- ^ a b Chapin JC, Hajjar KA (January 2015). "Fibrinolysis and the control of blood coagulation". Blood Reviews. 29 (1): 17–24. doi:10.1016/j.blre.2014.09.003. PMC 4314363. PMID 25294122.
- ^ Chan WS, Spencer FA, Ginsberg JS (April 2010). "Anatomic distribution of deep vein thrombosis in pregnancy". Canadian Medical Association Journal. 182 (7): 657–60. doi:10.1503/cmaj.091692. PMC 2855912. PMID 20351121.
- ^ Kim ES, Bartholomew JR. "Venous thromboembolism". Disease Management Project. Cleveland Clinic. Archived from the original on 23 February 2011. Retrieved 15 February 2011.
- ^ a b Saha P, Humphries J, Modarai B, Mattock K, Waltham M, Evans CE, et al. (March 2011). "Leukocytes and the natural history of deep vein thrombosis: current concepts and future directions". Arteriosclerosis, Thrombosis, and Vascular Biology. 31 (3): 506–12. doi:10.1161/ATVBAHA.110.213405. PMC 3079895. PMID 21325673.
- ^ Ro A, Kageyama N, Mukai T (June 2017). "Pathophysiology of venous thromboembolism with respect to the anatomical features of the deep veins of lower limbs: a review". Annals of Vascular Diseases. 10 (2): 99–106. doi:10.3400/avd.ra.17-00035. PMC 5579784. PMID 29034034.
- ^ a b Nicklas JM, Gordon AE, Henke PK (March 2020). "Resolution of deep venous thrombosis: proposed immune paradigms". International Journal of Molecular Sciences. 21 (6): 2080. doi:10.3390/ijms21062080. PMC 7139924. PMID 32197363.
- ^ López JA, Chen J (2009). "Pathophysiology of venous thrombosis". Thrombosis Research. 123 (Suppl 4): S30–34. doi:10.1016/S0049-3848(09)70140-9. PMID 19303501.
- ^ a b c Swystun LL, Liaw PC (August 2016). "The role of leukocytes in thrombosis". Blood. 128 (6): 753–62. doi:10.1182/blood-2016-05-718114. PMID 27354721.
- ^ a b Thålin C, Hisada Y, Lundström S, Mackman N, Wallén H (September 2019). "Neutrophil extracellular traps: villains and targets in arterial, venous, and cancer-associated thrombosis". Arteriosclerosis, Thrombosis, and Vascular Biology. 39 (9): 1724–38. doi:10.1161/ATVBAHA.119.312463. PMC 6703916. PMID 31315434.
- ^ Laridan E, Martinod K, De Meyer SF (February 2019). "Neutrophil Extracellular Traps in Arterial and Venous Thrombosis". Seminars in Thrombosis and Hemostasis. 45 (1): 86–93. doi:10.1055/s-0038-1677040. PMID 30634198. S2CID 58594612.
- ^ a b Myers DD (March 2015). "Pathophysiology of venous thrombosis". Phlebology. 30 (1 Suppl): 7–13. doi:10.1177/0268355515569424. PMID 25729062. S2CID 22467822.
- ^ a b "DDI/9290 clinical: D-dimer, plasma". Mayo Medical Laboratories. Archived from the original on 8 October 2012. Retrieved 27 August 2012.
- ^ Vedantham S, Goldhaber SZ, Kahn SR, Julian J, Magnuson E, Jaff MR, et al. (April 2013). "Rationale and design of the ATTRACT Study: a multicenter randomized trial to evaluate pharmacomechanical catheter-directed thrombolysis for the prevention of postthrombotic syndrome in patients with proximal deep vein thrombosis". American Heart Journal. 165 (4): 523–530.e3. doi:10.1016/j.ahj.2013.01.024. PMC 3612268. PMID 23537968.
- ^ Geersing GJ, Zuithoff NP, Kearon C, Anderson DR, Ten Cate-Hoek AJ, Elf JL, et al. (March 2014). "Exclusion of deep vein thrombosis using the Wells rule in clinically important subgroups: individual patient data meta-analysis". BMJ. 348 (7949): g1340. doi:10.1136/bmj.g1340. PMC 3948465. PMID 24615063.
- ^ a b c Pyzocha N (December 2019). "Diagnosing DVT in nonpregnant adults in the primary care setting". American Family Physician. 100 (12): 778–80. PMID 31845779.
- ^ a b c Stone J, Hangge P, Albadawi H, Wallace A, Shamoun F, Knuttien MG, et al. (December 2017). "Deep vein thrombosis: pathogenesis, diagnosis, and medical management". Cardiovascular Diagnosis and Therapy. 7 (Suppl 3): S276–84. doi:10.21037/cdt.2017.09.01. PMC 5778510. PMID 29399531.
- ^ a b c Wells PS, Ihaddadene R, Reilly A, Forgie MA (January 2018). "Diagnosis of venous thromboembolism: 20 years of progress". Annals of Internal Medicine. 168 (2): 131–40. doi:10.7326/M17-0291. PMID 29310137. S2CID 34220435.
- ^ a b Le Gal G, Righini M (June 2015). "Controversies in the diagnosis of venous thromboembolism". Journal of Thrombosis and Haemostasis. 13 (Suppl 1): S259–65. doi:10.1111/jth.12937. PMID 26149033.
- ^ a b Rahaghi FN, Minhas JK, Heresi GA (September 2018). "Diagnosis of deep venous thrombosis and pulmonary embolism: new imaging tools and modalities". Clinics in Chest Medicine. 39 (3): 493–504. doi:10.1016/j.ccm.2018.04.003. PMC 6317734. PMID 30122174.
- ^ Häggström, M (January 2019). "Subsartorial vessels as replacement names for superficial femoral vessels" (PDF). International Journal of Anatomy, Radiology and Surgery. 8 (1): AV01–02. doi:10.7860/IJARS/2019/40329:2458.
- ^ Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, et al. (April 2011). "Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association". Circulation. 123 (16): 1788–830. doi:10.1161/CIR.0b013e318214914f. PMID 21422387.
- ^ Cundiff DK, Manyemba J, Pezzullo JC (January 2006). Cundiff DK (ed.). "Anticoagulants versus non-steroidal anti-inflammatories or placebo for treatment of venous thromboembolism". The Cochrane Database of Systematic Reviews. 2006 (1): CD003746. doi:10.1002/14651858.CD003746.pub2. PMC 7389637. PMID 16437461.
- ^ Fleck D, Albadawi H, Wallace A, Knuttinen G, Naidu S, Oklu R (December 2017). "Below-knee deep vein thrombosis (DVT): diagnostic and treatment patterns". Cardiovascular Diagnosis and Therapy. 7 (Suppl 3): S134–39. doi:10.21037/cdt.2017.11.03. PMC 5778527. PMID 29399516.
- ^ a b c d e Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. (February 2016). "Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report". Chest. 149 (2): 315–52. doi:10.1016/j.chest.2015.11.026. PMID 26867832.
- ^ a b Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. (February 2012). "Antithrombotic therapy for VTE disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines". Chest. 141 (2 Suppl): e419S–96S. doi:10.1378/chest.11-2301. PMC 3278049. PMID 22315268.
- ^ Shikdar S, Vashisht R, Bhattacharya PT (2021). "International normalized ratio (INR)". StatPearls [Internet]. Treasure Island, FL. PMID 29939529.
- ^ Guyatt et al. 2012, p. 22S: 3.2.
- ^ Middeldorp S, Prins MH, Hutten BA (August 2014). "Duration of treatment with vitamin K antagonists in symptomatic venous thromboembolism". The Cochrane Database of Systematic Reviews. 2014 (8): CD001367. doi:10.1002/14651858.CD001367.pub3. PMC 7074008. PMID 25092359.
- ^ a b de Jong PG, Coppens M, Middeldorp S (August 2012). "Duration of anticoagulant therapy for venous thromboembolism: balancing benefits and harms on the long term". British Journal of Haematology. 158 (4): 433–41. doi:10.1111/j.1365-2141.2012.09196.x. PMID 22734929.
- ^ Witt DM, Nieuwlaat R, Clark NP, Ansell J, Holbrook A, Skov J, et al. (November 2018). "American Society of Hematology 2018 guidelines for management of venous thromboembolism: optimal management of anticoagulation therapy". Blood Advances. 2 (22): 3257–91. doi:10.1182/bloodadvances.2018024893. PMC 6258922. PMID 30482765.
- ^ Keeling D, Baglin T, Tait C, Watson H, Perry D, Baglin C, et al. (August 2011). "Guidelines on oral anticoagulation with warfarin – fourth edition". British Journal of Haematology. 154 (3): 311–24. doi:10.1111/j.1365-2141.2011.08753.x. PMID 21671894.
- ^ Douketis J, Tosetto A, Marcucci M, Baglin T, Cushman M, Eichinger S, et al. (October 2010). "Patient-level meta-analysis: effect of measurement timing, threshold, and patient age on ability of D-dimer testing to assess recurrence risk after unprovoked venous thromboembolism". Annals of Internal Medicine. 153 (8): 523–31. doi:10.7326/0003-4819-153-8-201010190-00009. PMID 20956709. S2CID 10659607.
- ^ Chatsis V, Visintini S (January 2017). Early mobilization for patients with venous thromboembolism: a review of clinical effectiveness and guidelines (Report). Ottawa, ON: Canadian Agency for Drugs and Technologies in Health. PMID 30303669.
- ^ Berntsen CF, Kristiansen A, Akl EA, Sandset PM, Jacobsen EM, Guyatt G, et al. (April 2016). "Compression Stockings for Preventing the Postthrombotic Syndrome in Patients with Deep Vein Thrombosis". The American Journal of Medicine. 129 (4): 447.e1–447.e20. doi:10.1016/j.amjmed.2015.11.031. PMID 26747198.
- ^ Wigle P, Hein B, Bernheisel CR (October 2019). "Anticoagulation: updated guidelines for outpatient management". American Family Physician. 100 (7): 426–34. PMID 31573167.
- ^ Wallace A, Albadawi H, Hoang P, Fleck A, Naidu S, Knuttinen G, et al. (December 2017). "Statins as a preventative therapy for venous thromboembolism". Cardiovascular Diagnosis and Therapy. 7 (Suppl 3): S207–18. doi:10.21037/cdt.2017.09.12. PMC 5778529. PMID 29399524.
- ^ a b c d e f g "Overview | Venous thromboembolic diseases: diagnosis, management and thrombophilia testing | Guidance | NICE". www.nice.org.uk. 26 March 2020. Retrieved 16 October 2024.
- ^ Enden T, Haig Y, Kløw NE, Slagsvold CE, Sandvik L, Ghanima W, et al. (January 2012). "Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial". Lancet. 379 (9810): 31–38. doi:10.1016/S0140-6736(11)61753-4. PMID 22172244. S2CID 21801157.
- ^ Haig Y, Enden T, Grøtta O, Kløw NE, Slagsvold CE, Ghanima W, et al. (February 2016). "Post-thrombotic syndrome after catheter-directed thrombolysis for deep vein thrombosis (CaVenT): 5-year follow-up results of an open-label, randomised controlled trial". The Lancet Haematology. 3 (2): e64–71. doi:10.1016/S2352-3026(15)00248-3. PMID 26853645.
- ^ a b Vedantham S, Goldhaber SZ, Julian JA, Kahn SR, Jaff MR, Cohen DJ, et al. (December 2017). "Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis". The New England Journal of Medicine. 377 (23): 2240–52. doi:10.1056/NEJMoa1615066. PMC 5763501. PMID 29211671.
- ^ Bhandari T (6 December 2017). "Clot-busting drugs not recommended for most patients with blood clots". Washington University School of Medicine. Retrieved 21 January 2020.
- ^ a b "Overview | Percutaneous mechanical thrombectomy for acute deep vein thrombosis of the leg | Guidance | NICE". www.nice.org.uk. 12 June 2019. Retrieved 16 October 2024.
- ^ Comerota AJ, Kearon C, Gu CS, Julian JA, Goldhaber SZ, Kahn SR, et al. (February 2019). "Endovascular thrombus removal for acute iliofemoral deep vein thrombosis". Circulation. 139 (9): 1162–73. doi:10.1161/CIRCULATIONAHA.118.037425. PMC 6389417. PMID 30586751.
- ^ Magnuson EA, Chinnakondepalli K, Vilain K, Kearon C, Julian JA, Kahn SR, et al. (October 2019). "Cost-effectiveness of pharmacomechanical catheter-directed thrombolysis versus standard anticoagulation in patients with proximal deep vein thrombosis: results from the ATTRACT trial". Circulation: Cardiovascular Quality and Outcomes. 12 (10): e005659. doi:10.1161/CIRCOUTCOMES.119.005659. PMC 6788761. PMID 31592728.
- ^ Jones MR, Prabhakar A, Viswanath O, Urits I, Green JB, Kendrick JB, et al. (June 2019). "Thoracic outlet syndrome: a comprehensive review of pathophysiology, diagnosis, and treatment". Pain and Therapy. 8 (1): 5–18. doi:10.1007/s40122-019-0124-2. PMC 6514035. PMID 31037504.
- ^ a b c Ijaopo R, Oguntolu V, DCosta D, Garnham A, Hobbs S (March 2016). "A case of Paget-Schroetter syndrome (PSS) in a young judo tutor: a case report". Journal of Medical Case Reports. 10: 63. doi:10.1186/s13256-016-0848-0. PMC 4797165. PMID 26987584.
- ^ Turner TE, Saeed MJ, Novak E, Brown DL (July 2018). "Association of inferior vena cava filter placement for venous thromboembolic disease and a contraindication to anticoagulation with 30-day mortality". JAMA Network Open. 1 (3): e180452. doi:10.1001/jamanetworkopen.2018.0452. PMC 6324296. PMID 30646021.
- ^ Moll S (9 May 2012). "What kind of doctor do I need?". Clot Connect. Archived from the original on 28 January 2020. Retrieved 28 January 2020.
- ^ Kitchen L, Lawrence M, Speicher M, Frumkin K (July 2016). "Emergency department management of suspected calf-vein deep venous thrombosis: a diagnostic algorithm". The Western Journal of Emergency Medicine. 17 (4): 384–90. doi:10.5811/westjem.2016.5.29951. PMC 4944794. PMID 27429688.
- ^ "Inferior Vena Cava (IVC) Filter Placement". Johns Hopkins Medicine. 2020. Retrieved 28 January 2020.
- ^ Partsch H, Blättler W (November 2000). "Compression and walking versus bed rest in the treatment of proximal deep venous thrombosis with low molecular weight heparin". Journal of Vascular Surgery. 32 (5): 861–69. doi:10.1067/mva.2000.110352. PMID 11054217.
- ^ a b Folsom AR, Cushman M (December 2020). "Exploring opportunities for primary prevention of unprovoked venous thromboembolism: ready for prime time?". Journal of the American Heart Association. 9 (23): e019395. doi:10.1161/JAHA.120.019395. PMC 7763794. PMID 33191841.
- ^ Li L, Zhang P, Tian JH, Yang K (December 2014). "Statins for primary prevention of venous thromboembolism". The Cochrane Database of Systematic Reviews. 2014 (12): CD008203. doi:10.1002/14651858.CD008203.pub3. PMC 11127252. PMID 25518837.
- ^ Kunutsor SK, Seidu S, Khunti K (February 2017). "Statins and primary prevention of venous thromboembolism: a systematic review and meta-analysis" (PDF). The Lancet Haematology. 4 (2): e83–e93. doi:10.1016/S2352-3026(16)30184-3. hdl:1983/5a398e70-6c7c-40bd-a8d4-53c24d84f1a2. PMID 28089655. S2CID 24036108.
- ^ Biere-Rafi S, Hutten BA, Squizzato A, Ageno W, Souverein PC, de Boer A, et al. (June 2013). "Statin treatment and the risk of recurrent pulmonary embolism". European Heart Journal. 34 (24): 1800–06. doi:10.1093/eurheartj/eht046. PMID 23396492.
- ^ Sobieraj DM, Lee S, Coleman CI, Tongbram V, Chen W, Colby J, et al. (May 2012). "Prolonged versus standard-duration venous thromboprophylaxis in major orthopedic surgery: a systematic review". Annals of Internal Medicine. 156 (10): 720–27. doi:10.7326/0003-4819-156-10-201205150-00423. PMID 22412039. S2CID 22797561.
- ^ Falck-Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S, et al. (February 2012). "Prevention of VTE in orthopedic surgery patients: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines". Chest. 141 (2 Suppl): e278S–e325S. doi:10.1378/chest.11-2404. PMC 3278063. PMID 22315265.
- ^ Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JA, et al. (February 2012). "Prevention of VTE in nonorthopedic surgical patients: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines". Chest. 141 (2 Suppl): e227S–77S. doi:10.1378/chest.11-2297. PMC 3278061. PMID 22315263.
- ^ a b Bates SM, Rajasekhar A, Middeldorp S, McLintock C, Rodger MA, James AH, et al. (November 2018). "American Society of Hematology 2018 guidelines for management of venous thromboembolism: venous thromboembolism in the context of pregnancy". Blood Advances. 2 (22): 3317–59. doi:10.1182/bloodadvances.2018024802. PMC 6258928. PMID 30482767.
- ^ Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO (February 2012). "VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines". Chest. 141 (2 Suppl): e691S–e736S. doi:10.1378/chest.11-2300. PMC 3278054. PMID 22315276.
- ^ a b Kahn SR, Lim W, Dunn AS, Cushman M, Dentali F, Akl EA, et al. (February 2012). "Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines". Chest. 141 (2 Suppl): e195S–e226S. doi:10.1378/chest.11-2296. PMC 3278052. PMID 22315261. See section 6.0, Long-Distance Travel
- ^ "New DVT guidelines: No evidence to support "economy-class syndrome"". American College of Chest Physicians. 7 February 2012. Archived from the original on 3 June 2016. Retrieved 10 February 2012.
oral contraceptives, sitting in a window seat, advanced age, and pregnancy increase DVT risk in long-distance travelers
- ^ Clarke MJ, Broderick C, Hopewell S, Juszczak E, Eisinga A (April 2021). "Compression stockings for preventing deep vein thrombosis in airline passengers". The Cochrane Database of Systematic Reviews. 2021 (4): CD004002. doi:10.1002/14651858.CD004002.pub4. PMC 8092568. PMID 33878207.
- ^ Galanaud JP, Monreal M, Kahn SR (April 2018). "Epidemiology of the post-thrombotic syndrome". Thrombosis Research. 164: 100–09. doi:10.1016/j.thromres.2017.07.026. PMID 28844444.
- ^ Galanaud JP, Righini M, Le Collen L, Douillard A, Robert-Ebadi H, Pontal D, et al. (January 2020). "Long-term risk of postthrombotic syndrome after symptomatic distal deep vein thrombosis: The CACTUS-PTS study". Journal of Thrombosis and Haemostasis. 18 (4): 857–64. doi:10.1111/jth.14728. PMID 31899848.
- ^ Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, et al. (November 2014). "2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism". European Heart Journal. 35 (43): 3033–69, 3069a–69k. doi:10.1093/eurheartj/ehu283. PMID 25173341.
- ^ Johannesen CD, Flachs EM, Ebbehøj NE, Marott JL, Jensen GB, Nordestgaard BG, et al. (January 2020). "Sedentary work and risk of venous thromboembolism". Scandinavian Journal of Work, Environment & Health. 46 (1): 69–76. doi:10.5271/sjweh.3841. PMID 31385593.
- ^ a b c Grosse SD, Nelson RE, Nyarko KA, Richardson LC, Raskob GE (January 2016). "The economic burden of incident venous thromboembolism in the United States: A review of estimated attributable healthcare costs". Thrombosis Research. 137: 3–10. doi:10.1016/j.thromres.2015.11.033. PMC 4706477. PMID 26654719.
- ^ a b Monagle P, Cuello CA, Augustine C, Bonduel M, Brandão LR, Capman T, et al. (November 2018). "American Society of Hematology 2018 Guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism". Blood Advances. 2 (22): 3292–316. doi:10.1182/bloodadvances.2018024786. PMC 6258911. PMID 30482766.
- ^ Januel JM, Chen G, Ruffieux C, Quan H, Douketis JD, Crowther MA, et al. (January 2012). "Symptomatic in-hospital deep vein thrombosis and pulmonary embolism following hip and knee arthroplasty among patients receiving recommended prophylaxis: a systematic review". JAMA. 307 (3): 294–303. doi:10.1001/jama.2011.2029. PMID 22253396.
- ^ Margaglione M, Grandone E (February 2011). "Population genetics of venous thromboembolism. A narrative review". Thrombosis and Haemostasis. 105 (2): 221–31. doi:10.1160/TH10-08-0510. PMID 20941456. S2CID 17552169.
- ^ "Blood types". American Red Cross. Archived from the original on 14 August 2012. Retrieved 15 August 2012.
- ^ a b Haskell R (10 January 2018). "Serena Williams on motherhood, marriage, and making her comeback". Vogue. Retrieved 22 January 2020.
- ^ Golemi I, Salazar Adum JP, Tafur A, Caprini J (August 2019). "Venous thromboembolism prophylaxis using the Caprini score". Disease-a-Month. 65 (8): 249–98. doi:10.1016/j.disamonth.2018.12.005. PMID 30638566. S2CID 58564402.
- ^ Perez AJ (17 February 2016). "Expert: Chris Bosh, like many, at risk of blood clot recurrence". USA Today. Retrieved 22 January 2020.
- ^ Martin J (4 May 2016). "Chris Bosh officially out as Heat make playoff push". CNN. Retrieved 22 January 2020.
- ^ Moisse K (2 March 2011). "Serena Williams Hospitalized After Pulmonary Embolism". ABC News. Retrieved 22 January 2020.
- ^ Andrews BL, Friedman Ross L (February 2021). "Black Women and Babies Matter". The American Journal of Bioethics. 21 (2): 93–95. doi:10.1080/15265161.2020.1861384. PMID 33534674. S2CID 231803661.
- ^ Pascarella L, Pappas TN (February 2013). "Phlebitis, pulmonary emboli and presidential politics: Richard M. Nixon's complicated deep vein thrombosis". The American Surgeon. 79 (2): 128–34. doi:10.1177/000313481307900222. PMID 23336651.
- ^ Frankel TC (11 September 2016). "Hillary Clinton has not been quick to share health information". The Washington Post. Retrieved 22 January 2020.
- ^ "Cheney diagnosed with deep-vein thrombosis". The Guardian. 6 March 2007. Retrieved 22 January 2020.
- ^ a b Rodriguez D (12 October 2018). "11 Celebrities Who Battled Deep Vein Thrombosis Risk". Everyday Health. Retrieved 22 January 2020.
- ^ "Coroner: Rapper Heavy D died of blood clot in lung". CNN. 27 December 2011. Retrieved 22 January 2020.
- ^ "My husband should be living today". TODAY. 3 March 2005. Retrieved 22 January 2020.
- ^ Eliot M (2006). Jimmy Stewart: A Biography. New York: Random House. p. 409. ISBN 978-1400052226.
- ^ a b c Goodman LR (October 2013). "In search of venous thromboembolism: the first 2913 years". American Journal of Roentgenology. 201 (4): W576-81. doi:10.2214/AJR.13.10604. PMID 24059395.
- ^ Galanaud JP, Laroche JP, Righini M (March 2013). "The history and historical treatments of deep vein thrombosis". Journal of Thrombosis and Haemostasis. 11 (3): 402–11. doi:10.1111/jth.12127. PMID 23297815.
- ^ a b c Kumar DR, Hanlin E, Glurich I, Mazza JJ, Yale SH (December 2010). "Virchow's contribution to the understanding of thrombosis and cellular biology". Clinical Medicine & Research. 8 (3–4): 168–72. doi:10.3121/cmr.2009.866. PMC 3006583. PMID 20739582.
- ^ a b Bagot CN, Arya R (October 2008). "Virchow and his triad: a question of attribution". British Journal of Haematology. 143 (2): 180–90. doi:10.1111/j.1365-2141.2008.07323.x. PMID 18783400.
- ^ Dalen 2003, p. 3.
- ^ Line BR, Peters TL, Keenan J (January 1997). "Diagnostic test comparisons in patients with deep venous thrombosis" (PDF). Journal of Nuclear Medicine. 38 (1): 89–92. PMID 8998158.
- ^ Dalen 2003, p. 2.
- ^ a b Ornstein C, Jones RG (7 January 2015). "The drugs that companies promote to doctors are rarely breakthroughs". The New York Times.
- ^ a b Franchini M, Mannucci PM (September 2016). "Direct oral anticoagulants and venous thromboembolism". European Respiratory Review. 25 (141): 295–302. doi:10.1183/16000617.0025-2016. PMC 9487211. PMID 27581829.
- ^ Dasta JF, Pilon D, Mody SH, Lopatto J, Laliberté F, Germain G, et al. (February 2015). "Daily hospitalization costs in patients with deep vein thrombosis or pulmonary embolism treated with anticoagulant therapy". Thrombosis Research. 135 (2): 303–10. doi:10.1016/j.thromres.2014.11.024. PMID 25555319.
- ^ Ruppert A, Steinle T, Lees M (2011). "Economic burden of venous thromboembolism: a systematic review". Journal of Medical Economics. 14 (1): 65–74. doi:10.3111/13696998.2010.546465. PMID 21222564.
- ^ Dobesh PP (August 2009). "Economic burden of venous thromboembolism in hospitalized patients". Pharmacotherapy. 29 (8): 943–53. doi:10.1592/phco.29.8.943. PMID 19637948. S2CID 8966676.
- ^ Fernandez MM, Hogue S, Preblick R, Kwong WJ (2015). "Review of the cost of venous thromboembolism". ClinicoEconomics and Outcomes Research. 7: 451–62. doi:10.2147/CEOR.S85635. PMC 4559246. PMID 26355805.
- ^ Metz AK, Diaz JA, Obi AT, Wakefield TW, Myers DD, Henke PK (2018). "Venous thrombosis and post-thrombotic syndrome: from novel biomarkers to biology". Methodist DeBakey Cardiovascular Journal. 14 (3): 173–81. doi:10.14797/mdcj-14-3-173. PMC 6217569. PMID 30410646.
Cited literature
- Dalen JE (2003). Venous thromboembolism. CRC Press. ISBN 978-0824756451.
- Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ (February 2012). "Executive summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines". Chest. 141 (2 Suppl): 7S–47S. doi:10.1378/chest.1412S3. PMC 3278060. PMID 22315257.

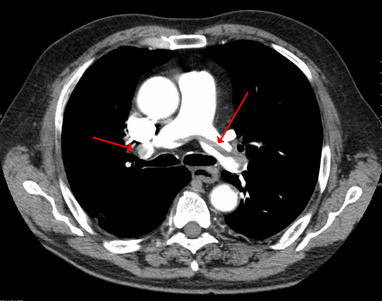
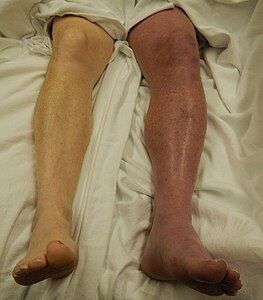
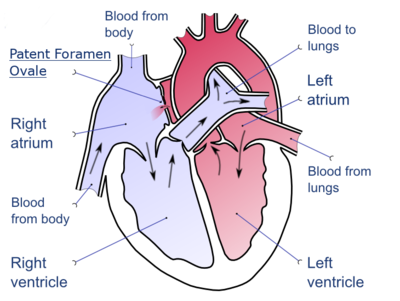
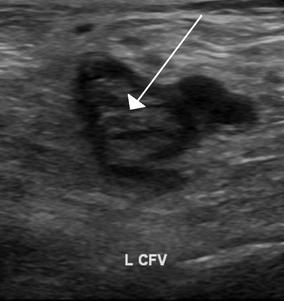
![Doppler ultrasonography showing absence of flow and hyperechogenic content in a clotted femoral vein (labeled subsartorial[h]) distal to the branching point of the deep femoral vein. When compared to this clot, clots that instead obstruct the common femoral vein (proximal to this branching point) cause more severe effects due to impacting a significantly larger portion of the leg.[122]](/uploads/wikipedia/commons/thumb/4/4f/Ultrasonography_of_deep_vein_thrombosis_of_the_femoral_vein_-annotated.jpg/459px-Ultrasonography_of_deep_vein_thrombosis_of_the_femoral_vein_-annotated.jpg?auto=webp)


![After treatment with catheter-directed thrombolysis, blood flow in the axillary and subclavian vein were significantly improved. Afterwards, a first rib resection allowed decompression. This reduces the risk of recurrent DVT and other sequelae from thoracic outlet compression.[147]](/uploads/wikipedia/commons/6/6e/A-case-of-Paget-Schroetter-syndrome-%28PSS%29-in-a-young-judo-tutor-a-case-report-13256_2016_848_Fig2_HTML.jpg?auto=webp)