Chromium(VI) oxide peroxide
| |
| Names | |
|---|---|
| IUPAC name
Chromium(VI) oxide diperoxide
| |
Other names
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| 101104 | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CrO(O2)2 | |
| Molar mass | 131.991 g·mol−1 |
| Appearance | Dark blue |
| soluble (decomposes) | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
May suddenly explode if unstabilized/dry, toxic and create highly carcinogenic chromium fumes. |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
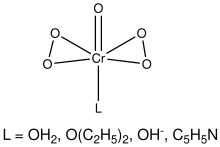
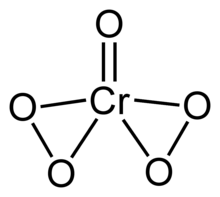
Chromium(VI) oxide peroxide is the name given to a collection of chromium coordination complexes. They have the formula CrO(O2)2L where L is a ligand. These species are dark blue and often labile. They all feature oxo ligand and two peroxo ligands, with the remaining coordination sites occupied by water, hydroxide, ether, or other Lewis bases.[1]
Preparation and properties
[edit]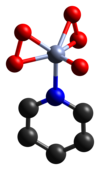
Chromium(VI) oxide peroxide is formed by the addition of acidified hydrogen peroxide solutions to solutions of metal chromates or dichromates, such as sodium chromate or potassium dichromate. The generally yellow chromates or orange dichromates turn to dark blue as "chromium(VI) oxide peroxide" forms:
- CrO2−4 + 2 H2O2 + H+ → [CrO(O2)2OH]− + 3 H2O
The structure of the pyridine complex has been determined crystallographically.[2] Adducts with other N-heterocycles have also been characterized similarly.[3]
Aqueous chromium(VI) oxide peroxide decomposes in a few seconds, turning green as chromium(III) compounds are formed.[4]
- 2 CrO(O2)2 + 7 H2O2 + 6 H+ → 2 Cr3+ + 10 H2O + 7 O2
Stable adducts of the type CrO(O2)2L include those with L = diethyl ether, 1-butanol, ethyl acetate, or amyl acetate. They form by adding a layer of the organic solvent above the chromate/dichromate solution and shaking during the addition of hydrogen peroxide.
The etherate, bis(pyridine) and pyridine complexes of this compound have been found to be effective oxidants in organic chemistry.[5]
CrO5 is sometimes said to have a "Butterfly-like" Structure due to its arrangement according to Valence Bond Theory.
Gallery
[edit]-
An aqueous solution of "chromium peroxide"
-
A dilute solution of "chromium peroxide"
-
"chromium(VI) oxide peroxide" in ether phase (above) and chromium(III) aqueous solution (below).
See also
[edit]- Tetraperoxochromate - an similarly-synthesized analogous chromium(V) peroxide complex
References
[edit]- ^ Gili, Pedro; Mederos, Alfredo; Lorenzo-Luis, Pablo A.; de la Rosa, Eduardo Medina; Muñoz, Alfonso (2002). "On the interaction of compounds of chromium(VI) with hydrogen peroxide. A study of chromium(VI) and (V) peroxides in the acid–basic pH range". Inorganica Chimica Acta. 331: 16–24. doi:10.1016/S0020-1693(01)00747-2.
- ^ Stomberg, Rolf (1962). "Crystal Structure of Peroxochromates, CrO5⋅C5H5N". Nature. 196 (4854): 570–571. doi:10.1038/196570b0. S2CID 4187294.
- ^ Stomberg, Rolf; Ainalem, Ing-Britt; Johansson, Gunnar; Tolboe, O.; Paasivirta, Jaakko (1968). "The Crystal Structure of two Modifications of Oxidodiperoxido-2,2'-dipyridylchromium(VI), [CrO(O2)2(C10H8N2)]". Acta Chemica Scandinavica. 22: 1439–1451. doi:10.3891/acta.chem.scand.22-1439.
- ^ Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils; (1985). "Chromium" (in German). Lehrbuch der Anorganischen Chemie (91–100 ed.). Walter de Gruyter. pp. 1081–1095. ISBN 3-11-007511-3."
- ^ Firouzabadi, H.; Iranpoor, N.; Kiaeezadeh, F.; Toofan, J. (1986). "Chromium(VI) based oxidants-1 Chromium peroxide complexes as versatile, mild, and efficient oxidants in organic synthesis". Tetrahedron. 42: 719. doi:10.1016/S0040-4020(01)87476-7.
External links
[edit]- Experimental details and photo (in German)
- Losing fingers to chemistry on YouTube (8 November 2012). Professor Martyn Poliakoff of the University of Nottingham demonstrates the synthesis on Periodic Videos.



