Cerium(III) fluoride

| |
| |
| Names | |
|---|---|
| IUPAC name
Cerium(III) fluoride
| |
| Other names
Cerium trifluoride
| |
| Identifiers | |
| ChemSpider | |
| ECHA InfoCard | 100.028.947 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| Properties | |
| CeF3 | |
| Molar mass | 197.12 g/mol |
| Density | 6.16 g/cm3 (at 20 °C) |
| Melting point | 1,460 °C (2,660 °F; 1,730 K)[1] |
| Related compounds | |
Related compounds
|
Lanthanum trifluoride |
| Hazards | |
| GHS labelling: | |
 
| |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Cerium(III) fluoride (or cerium trifluoride), CeF3, is an ionic compound of the rare earth metal cerium and fluorine.
It appears as a mineral in the form of fluocerite-(Ce) - a very rare mineral species related mainly to pegmatites and rarely to oxidation zones of some polymetallic ore deposits.[2][3] CeF3 may be used as a Faraday rotator material in the visible, near-infrared and mid-infrared spectral range.[4][5]
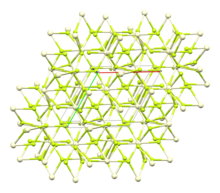
Structure
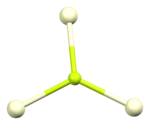
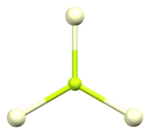
[edit]The crystal structure of cerium(III) fluoride is described as the LaF3 or tysonite structure.[6] It contains 9-coordinate cerium ions that adopt an approximately tricapped trigonal prismatic coordination geometry,[7] although it can be considered 11-coordinate if two more distant fluorides are considered part of the cerium coordination environment.[6] The three crystallographically independent fluoride ions are 3-coordinate and range in geometry from trigonal planar to pyramidal.[6]
| Cerium coordination | Fluorine F1 coordination | Fluorine F2 coordination | Fluorine F3 coordination |
|---|---|---|---|

|
|

|
|
References
[edit]- ^ Holleman-Wiberg, 102. edition, p. 1942[full citation needed]
- ^ "Fluocerite-(Ce)".
- ^ "List of Minerals". 21 March 2011.
- ^ Vojna, David; Yasuhara, Ryo; Slezák, Ondřej; Mužík, Jiří; Lucianetti, Antonio; Mocek, Tomáš (2017). "Verdet constant dispersion of CeF3 in the visible and near-infrared spectral range". Optical Engineering. 56 (6): 067105. Bibcode:2017OptEn..56f7105V. doi:10.1117/1.oe.56.6.067105. S2CID 125990210.
- ^ Vojna, David; Slezák, Ondřej; Yasuhara, Ryo; Furuse, Hiroaki; Lucianetti, Antonio; Mocek, Tomáš (2020). "Faraday Rotation of Dy2O3, CeF3 and Y3Fe5O12 at the Mid-Infrared Wavelengths". Materials. 13 (23): 5324. Bibcode:2020Mate...13.5324V. doi:10.3390/ma13235324. PMC 7727863. PMID 33255447.
- ^ a b c Wells, A. F. (1984). Structural Inorganic Chemistry (5th ed.). Oxford University Press. pp. 420–421. ISBN 978-0-19-965763-6.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 1240–1241. ISBN 978-0-08-037941-8.
- ^ Cheetham, A. K.; Fender, B. E. F.; Fuess, H.; Wright, A. F. (1976). "A powder neutron diffraction study of lanthanum and cerium trifluorides". Acta Crystallogr. B. 32: 94–97. doi:10.1107/S0567740876002380.

