Carfentrazone
| |
| Names | |
|---|---|
| IUPAC name
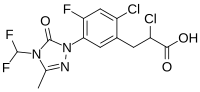
2-Chloro-3-[2-chloro-5-[4-(difluoromethyl)-3-methyl-5-oxo-1,2,4-triazol-1-yl]-4-fluorophenyl]propanoic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.127.279 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C13H10Cl2F3N3O3 | |
| Molar mass | 384.14 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Carfentrazone is an aryl triazoline herbicide, used for pre-crop-establishment control of broadleaf weeds, including marshmallow, annual nettles, and volunteer cotton, and frequently in tank mixtures with knock-down herbicides, such as glyphosate. Under the system of HRAC classification it is a Group 14 (numeric), E (global) or G (Australian), since it acts by membrane disruption, through the inhibition of protoporphyrinogen oxidase. Carfentrazone is used on crops, fallows, forest plantations, industrial, horticultural and commercial areas.[1]
Carfentrazone is used in Australia,[1] the United Kingdom, the European Union, and the United States. It was introduced in 1997.[2]
Mechanism and effects
[edit]Affected weeds rapidly absorb carfentrazone through foliage,[1] and show symptoms within hours, of desiccation, necrosis and death. It is often applied at 75 mL/ha in Australia, or less if part of a knockdown mixture with glyphosate, due to carfentrazone's prevention of weeds' metabolism, which reduces the translocation and effectiveness of glyphosate. Effectiveness is best when applied with much water, and reduced by high temperatures, sunlight and muddy or saline mix-water.[3]
Safety
[edit]Carfentrazone is toxic to aquatic life, both in acute and chronic toxicities.[4] In mammals, carfentrazone has a low toxicity, with an LD50 of over 5000 mg/kg, tested orally in rats. For birds, carfentrazone is also of low toxicity.[2]
References
[edit]- ^ a b c "Carfentrazone 240 EC Leaflet" (PDF). www.4farmers.com.au. 4Farmers Australia.
- ^ a b Lewis, Kathleen A.; Tzilivakis, John; Warner, Douglas J.; Green, Andrew (2016). "An international database for pesticide risk assessments and management". Human and Ecological Risk Assessment: An International Journal. 22 (4): 1050–1064. Bibcode:2016HERA...22.1050L. doi:10.1080/10807039.2015.1133242. hdl:2299/17565.
- ^ "Carfentrazone 240 EC Infosheet" (PDF). www.4farmers.com.au. 4Farmers Australia.
- ^ "4F Carfentrazone 240 EC SDS" (PDF). www.4farmers.com.au. 4Farmers Australia.
Links
[edit]- Carfentrazone in the Pesticide Properties DataBase (PPDB)
