Lecanemab
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Humanized |
| Target | Amyloid beta |
| Clinical data | |
| Trade names | Leqembi |
| Other names | BAN2401, lecanemab-irmb |
| AHFS/Drugs.com | Monograph |
| License data | |
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C6544H10088N1744O2032S46 |
| Molar mass | 147181.62 g·mol−1 |
Lecanemab, sold under the brand name Leqembi, is a monoclonal antibody medication used for the treatment of Alzheimer's disease.[2][4] Lecanemab is an amyloid beta-directed antibody.[2] It is given via intravenous infusion to patients with mild cognitive impairment or mild dementia.[2] In clinical trials, it demonstrated modest efficacy in reducing relative cognitive decline compared to placebo.[5] The most common side effects of lecanemab include headache, infusion-related reactions, and amyloid-related imaging abnormalities, a side effect known to occur with the class of antibodies targeting amyloid.[6]
Lecanemab was jointly developed by Eisai and Biogen. It was granted accelerated approval for medical use in the United States in January 2023,[7] and fully approved by the FDA in July 2023.[4][8]
Medical uses
[edit]Lecanemab is indicated for the treatment of Alzheimer's disease in people who have mild cognitive impairment or mild dementia, but not in people who already have moderate or severe dementia.[2][4][6]
Efficacy
[edit]In a phase III clinical trial of 1,795 patients aged 50 to 90 years old with early-stage Alzheimer’s disease, lecanemab slowed clinical decline by 27% after 18 months of treatment compared with those who received a placebo.[9][10] The mean CDR-SOB score at baseline was approximately 3.2 among the study population, and the mean change from baseline after 18 months was +1.21 with lecanemab and +1.66 with placebo. (For the comparison, CDR-SOB score is 0 for the Normal level, 0.5–2.5 for Questionable impairment, 3.0–4.0 for Very mild dementia, 4.5–9.0 for Mild dementia, 9.5–15.5 for Moderate dementia, and 16.0–18.0 for Severe dementia.)[11] The authors concluded "Lecanemab reduced markers of amyloid in early Alzheimer’s disease and resulted in moderately less decline on measures of cognition and function than placebo at 18 months but was associated with adverse events."[10]
Adverse effects
[edit]Lecanemab may cause amyloid-related imaging abnormalities (ARIA). ARIA is often asymptomatic, but serious and life-threatening events rarely may occur. ARIA most commonly presents as temporary swelling of the brain that usually resolves over time and may be accompanied by small spots of bleeding in or on the surface of the brain, though some people may have symptoms such as headache, confusion, dizziness, vision changes, nausea and seizure.[4][6] Compared to placebo, all doses of the drug caused accelerated brain shrinkage.[12]
Pharmacology
[edit]Mechanism of action
[edit]
Lecanemab is a monoclonal antibody consisting of the humanized version[13] of a mouse antibody, mAb158, that recognizes protofibrils and prevents amyloid beta deposition in animal models of Alzheimer's disease.[14]
History
[edit]In July 2022, the US Food and Drug Administration (FDA) accepted an application for accelerated approval for lecanemab.[15]
In September 2022, Biogen announced[15][16] positive results from an ongoing phase III clinical trial.[17][18]
In November 2022, it was announced that the drug was a success in clinical trials, and exceeded its goal in reaching primary endpoints.[19]
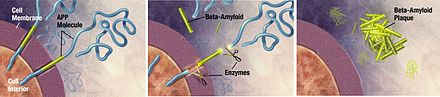
The efficacy of lecanemab was evaluated in a double-blind, placebo-controlled, parallel-group, dose-finding study of 856 participants with Alzheimer's disease.[4] Treatment was initiated in participants whose disease was in the stage of mild cognitive impairment or mild dementia and who had confirmed presence of amyloid beta pathology.[4] Participants receiving the treatment showed significant dose- and time-dependent reduction of amyloid beta plaque: Those receiving the approved dose of lecanemab, 10 milligrams/kilogram every two weeks, had a statistically significant reduction in brain amyloid plaque from baseline to week 79 compared with those receiving a placebo, who had no reduction of amyloid beta plaque.[4]
The FDA approved lecanemab in January 2023, via the accelerated approval pathway for the treatment of Alzheimer's disease.[4] The FDA granted the application for lecanemab fast track, priority review, and breakthrough therapy designations.[4] The approval of Leqembi was granted to Eisai R&D Management Co., Ltd.[4] In July 2023, the FDA converted lecanemab to traditional approval.[6]
Efficacy of lecanemab was evaluated using the results of Study 301 (CLARITY AD), a phase III randomized, controlled clinical trial.[6] Study 301 was a multicenter, randomized, double-blind, placebo-controlled, parallel-group study that enrolled 1,795 participants with Alzheimer's disease.[6] Treatment was initiated in participants with mild cognitive impairment or mild dementia stage of disease and confirmed presence of amyloid beta pathology.[6] Participants were randomized in a 1:1 ratio to receive placebo or lecanemab at a dose of 10 milligrams (mg)/kilograms (kg), once every two weeks.[6] Lecanemab demonstrated a statistically significant and clinically meaningful reduction of decline from baseline to 18 months on the primary endpoint, the Clinical Dementia Rating Scale Sum of Boxes score, compared to placebo.[6] Statistically significant differences between treatment groups were also demonstrated on all secondary endpoints, which included the Alzheimer's Disease Assessment Scale Cognitive Subscale 14, and the Alzheimer's Disease Cooperative Study–Activities of Daily Living Scale for Mild Cognitive Impairment.[6]
Society and culture
[edit]Legal status
[edit]Australia
[edit]In October 2024, the Australian Therapeutic Goods Administration (TGA) decided not to register lecanemab.[20]
European Union
[edit]In July 2024, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) recommended the refusal of the marketing authorization for lecanemab. The manufacturer requested re-examination.[21]
In November 2024, after re-examining its initial opinion, the CHMP recommended granting a marketing authorization to lecanemab (Leqembi) for treating mild cognitive impairment (memory and thinking problems) or mild dementia due to Alzheimer's disease (early Alzheimer's disease) in people who have only one or no copy of ApoE4, a certain form of the gene for the protein apolipoprotein E.[21][22]
United Kingdom
[edit]In August 2024, the Medicines and Healthcare products Regulatory Agency (MHRA) granted marketing authorization for England, Scotland, and Wales. It may not be used in people with two copies of the ApoE4 allele. Statistically, these people develop Alzheimer's disease more frequently and earlier, but also have a particularly high incidence of ARIA when treated with lecanemab. According to the MHRA, the risk outweighs the benefit for these people and genetic testing is recommended before starting treatment.[23][24][25]
The English branch of the National Health Service (NHS) announced it would not cover the costs of treatment, as according to draft guidance from the National Institute for Health and Care Excellence (NICE), the small benefit does not justify the cost of treatment.[23][24][25]
Drugs in [Northern Ireland are regulated by the European Medicines Agency (EMA) after Brexit in accordance with the Northern Ireland Protocol.[25]
United States
[edit]In January 2023, the FDA granted accelerated approval for lecanemab.[4][26] In July 2023, the FDA converted lecanemab to traditional approval.[6]
Reception
[edit]In October 2023, lecanemab was designated as a Do Not Use drug by Public Citizen's Health Research Group.[27] It had urged the FDA not to approve it, arguing that there were serious safety concerns and very small treatment benefits.[27]
Economics
[edit]Lecanemab pricing is US$26,500 per year,[28] with a company-estimated "per-patient societal value" of $37,600.[29] However, cost-effectiveness analysis by the Institute for Clinical and Economic Review (ICER) concluded that a broad range of $8,900 to $21,500 would be appropriate.[30] According to an estimate by the manufacturer, Eisai, about 85% of eligible people with early-Alzheimer's in the United States are covered by Medicare.[29]
After reviewing the clinical evidence and considering the treatments' other potential benefits, disadvantages, and contextual considerations noted above, the California Technology Assessment Forum unanimously concluded that lecanemab represents "low" long-term value of money.[30] At lecanemab's net price, approximately 5% of the 1.4 million people in the US eligible for Alzheimer's disease treatment that targets beta-amyloid could be treated within five years without crossing the ICER potential budget impact threshold of $777 million per year.[30] As a result, ICER issued an access and affordability alert for lecanemab in the management of Alzheimer's disease. This alert indicates that the health care costs of the treatment might stress the health system in the short term, resulting in the displacement of other services and a rapid increase in insurance costs.[30]
Names
[edit]Lecanemab is the international nonproprietary name.[31]
Research
[edit]Lecanemab was jointly developed by the companies Eisai and Biogen and is in clinical trials for the treatment of Alzheimer's disease.[32]
It has shown statistically significant but minor effectiveness, with studies suggesting a modest decrease in cognitive decline in Alzheimer's participants compared with a control group given a placebo instead.[33]
According to a phase III clinical trial (n = 1795), lecanemab has been associated with both ARIA-E (cerebral edema) and ARIA-H (microhaemorrhages, or small haemorrhages, and hemosiderosis) sub-types.[10] Mild to moderate infusion-related reactions may also occur.[10]
References
[edit]- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ a b c d e "Leqembi – lecanemab injection, solution". DailyMed. 11 January 2023. Archived from the original on 15 January 2023. Retrieved 21 January 2023.
- ^ "Update on Regulatory Review of Lecanemab for Early Alzheimer's Disease in Australia". Biogen (Press release). 16 October 2024. Retrieved 14 November 2024.
- ^ a b c d e f g h i j k "FDA Grants Accelerated Approval for Alzheimer's Disease Treatment" (Press release). U.S. Food and Drug Administration (FDA). 6 January 2023. Archived from the original on 7 January 2023. Retrieved 7 January 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Kwon D (22 August 2024). "Debate rages over Alzheimer's drug lecanemab as UK limits approval". Nature. doi:10.1038/d41586-024-02720-y.
- ^ a b c d e f g h i j k "FDA Converts Novel Alzheimer's Disease Treatment to Traditional Approval". U.S. Food and Drug Administration (FDA) (Press release). 6 July 2023. Archived from the original on 6 July 2023. Retrieved 6 July 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Drug Approval Package: Leqembi". U.S. Food and Drug Administration (FDA). 6 February 2023. Archived from the original on 9 July 2023. Retrieved 8 July 2023.
- ^ "Lecanemab Summary Review" (PDF). Center for Drug Evaluation and Research (CDER). U.S. Food and Drug Administration (FDA). Archived (PDF) from the original on 7 January 2023. Retrieved 7 January 2023.
- ^ "Lecanemab, the New Alzheimer's Treatment: 3 Things To Know". Yale Medicine. Archived from the original on 7 December 2023. Retrieved 7 December 2023.
- ^ a b c d van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, et al. (January 2023). "Lecanemab in Early Alzheimer's Disease". The New England Journal of Medicine. 388 (1). Massachusetts Medical Society: 9–21. doi:10.1056/nejmoa2212948. PMID 36449413. S2CID 254094094.
- ^ O'Bryant SE, Waring SC, Cullum CM, Hall J, Lacritz L, Massman PJ, et al. (August 2008). "Staging dementia using Clinical Dementia Rating Scale Sum of Boxes scores: a Texas Alzheimer's research consortium study". Archives of Neurology. 65 (8): 1091–1095. doi:10.1001/archneur.65.8.1091. PMC 3409562. PMID 18695059.
- ^ Liu KY, Villain N, Ayton S, Ackley SF, Planche V, Howard R, et al. (2 May 2023). "Key questions for the evaluation of anti-amyloid immunotherapies for Alzheimer's disease". Brain Communications. 5 (3): fcad175. doi:10.1093/braincomms/fcad175. PMC 10306158. PMID 37389302.
- ^ Lannfelt L, Möller C, Basun H, Osswald G, Sehlin D, Satlin A, et al. (2014). "Perspectives on future Alzheimer therapies: amyloid-β protofibrils – a new target for immunotherapy with BAN2401 in Alzheimer's disease". Alzheimer's Research & Therapy. 6 (2): 16. doi:10.1186/alzrt246. PMC 4054967. PMID 25031633.
- ^ Söllvander S, Nikitidou E, Gallasch L, Zyśk M, Söderberg L, Sehlin D, et al. (March 2018). "The Aβ protofibril selective antibody mAb158 prevents accumulation of Aβ in astrocytes and rescues neurons from Aβ-induced cell death". Journal of Neuroinflammation. 15 (1): 98. doi:10.1186/s12974-018-1134-4. PMC 5875007. PMID 29592816.
- ^ a b "Lecanemab Confirmatory Phase 3 Clarity Ad Study Met Primary Endpoint, Showing Highly Statistically Significant Reduction of Clinical Decline in Large Global Clinical Study of 1,795 Participants With Early Alzheimer's Disease" (Press release). Biogen. 27 September 2022. Archived from the original on 27 September 2022. Retrieved 28 September 2022.
- ^ Robbins R, Belluck P (27 September 2022). "Alzheimer's Drug Slows Cognitive Decline in Key Study". The New York Times. Archived from the original on 28 September 2022. Retrieved 28 September 2022.
- ^ "A Study to Confirm Safety and Efficacy of Lecanemab in Participants With Early Alzheimer's Disease (Clarity AD)". ClinicalTrials.gov. 11 July 2022. Archived from the original on 28 September 2022. Retrieved 28 September 2022.
- ^ Sample I (22 November 2022). "'This looks like the real deal': Are we inching closer to a treatment for Alzheimer's?". The Guardian. Archived from the original on 10 January 2023.
- ^ Gallagher J (30 November 2022). "Alzheimer's drug lecanemab hailed as momentous breakthrough". BBC News Online. Archived from the original on 2 December 2022. Retrieved 30 November 2022.
- ^ "TGA's decision to not register lecanemab (LEQEMBI)". Therapeutic Goods Administration (TGA) (Press release). 15 October 2024. Retrieved 14 November 2024.
- ^ a b "Leqembi". European Medicines Agency (EMA). 5 August 2024. Archived from the original on 27 July 2024. Retrieved 22 August 2024.
- ^ "Leqembi recommended for treatment of early Alzheimer's disease". European Medicines Agency (EMA). 14 November 2024. Retrieved 14 November 2024. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ a b Walsh F (22 August 2024). "Lecanemab: First drug to slow Alzheimer's too costly for NHS". www.bbc.com. Archived from the original on 23 August 2024. Retrieved 23 August 2024.
- ^ a b "Lecanemab licensed for adult patients in the early stages of Alzheimer's disease". Medicines and Healthcare products Regulatory Agency. 22 August 2024. Archived from the original on 23 August 2024. Retrieved 23 August 2024.
- ^ a b c Taylor E (22 August 2024). "New Alzheimer's treatment, lecanemab, makes the headlines: what's next?". Alzheimer's Research UK. Archived from the original on 23 August 2024. Retrieved 23 August 2024.
- ^ Howard J, Goodman B (6 January 2023). "Alzheimer's drug lecanemab receives accelerated approval amid safety concerns". CNN. Archived from the original on 6 January 2023. Retrieved 11 January 2023.
- ^ a b ""Lecanemab for Alzheimer's Disease: Do Not Use"". Worst Pills, Best Pills News. Vol. 29, no. 10. Public Citizen. 1 October 2023.
- ^ "Eisai's Approach To U.S. Pricing For Leqembi (Lecanemab), a Treatment For Early Alzheimer's Disease, Sets Forth Our Concept Of "Societal Value Of Medicine" In Relation To "Price Of Medicine"" (Press release). Eisai Inc. 6 January 2023. Archived from the original on 7 January 2023. Retrieved 7 January 2023 – via PR Newswire.
- ^ a b Bell J (6 February 2023). "Eisai gives first glimpse into Alzheimer's drug launch". BiopharmaDive. Archived from the original on 12 February 2023. Retrieved 11 February 2023.
- ^ a b c d "ICER Publishes Final Evidence Report on Lecanemab for Alzheimer's Disease" (Press release). Institute for Clinical and Economic Review. 17 April 2023. Archived from the original on 7 May 2023. Retrieved 7 May 2023.
- ^ World Health Organization (2020). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 84". WHO Drug Information. 34 (3). hdl:10665/340680.
- ^ Clinical trial number NCT01767311 for "Study to Evaluate Safety, Tolerability, and Efficacy of BAN2401 in Subjects With Early Alzheimer's Disease" at ClinicalTrials.gov
- ^ Devlin H (28 September 2022). "Success of experimental Alzheimer's drug hailed as 'historic moment'". The Guardian. Archived from the original on 28 September 2022.
Further reading
[edit]- Tolar M, Abushakra S, Hey JA, Porsteinsson A, Sabbagh M (August 2020). "Aducanumab, gantenerumab, BAN2401, and ALZ-801-the first wave of amyloid-targeting drugs for Alzheimer's disease with potential for near term approval". Alzheimer's Research & Therapy. 12 (1): 95. doi:10.1186/s13195-020-00663-w. PMC 7424995. PMID 32787971.
- Villain N, Planche V, Levy R (December 2022). "High-clearance anti-amyloid immunotherapies in Alzheimer's disease. Part 1: Meta-analysis and review of efficacy and safety data, and medico-economical aspects". Revue Neurologique. 178 (10): 1011–1030. doi:10.1016/j.neurol.2022.06.012. PMID 36184326.
