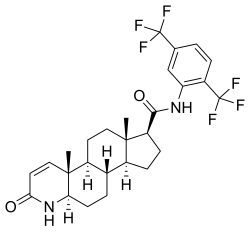
Dutasteride
 | |
| Clinical data | |
|---|---|
| Trade names | Avodart, others |
| Other names | GG-745; GI-198745; GI-198745X; N-[2,5-Bis(trifluoromethyl)phenyl]-3-oxo-4-aza-5α-androst-1-ene-17β-carboxamide |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603001 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | 5α-Reductase inhibitor |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 60%[1] |
| Protein binding | 99%[1] |
| Metabolism | Liver (CYP3A4)[1] |
| Metabolites | • 4'-Hydroxydutasteride[1] • 6'-Hydroxydutasteride[1] • 1,2-Dihydrodutasteride[1] (All three active)[1] |
| Elimination half-life | 4–5 weeks[2][3] |
| Excretion | Feces: 40% (metabolites)[1] Urine: 5% (unchanged)[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.166.372 |
| Chemical and physical data | |
| Formula | C27H30F6N2O2 |
| Molar mass | 528.539 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.346 g/cm3 at 294 K (calculated) [4] |
| |
| |
| (verify) | |
Dutasteride, sold under the brand name Avodart among others, is a medication primarily used to treat the symptoms of a benign prostatic hyperplasia (BPH), an enlarged prostate not associated with cancer. A few months may be required before benefits occur.[5] It is also used for scalp hair loss in men and as a part of hormone therapy in transgender women.[6][7] It is usually taken by mouth.[8][9][5]
The most commonly reported side effects of dutasteride, although rare, include sexual dysfunction and depression.[8] In the largest available study of 6,729 men with BPH, 9% experienced erectile dysfunction (compared to 5.7% treated with a placebo), 3.3% experienced decreased sex drive (vs 1.6% of placebo), and 1.9% had enlarged breasts (vs 1% of placebo).[10][11] Exposure during pregnancy is specifically contraindicated because antiandrogens such as dutasteride have been shown to interfere with the sexual development of male fetuses.[3][8]
Dutasteride was patented in 1993 by GlaxoSmithKline and was approved for medical use in 2001.[12][8] In the United States and elsewhere, it is available as a generic medication.[5] In 2018, it was the 291st-most commonly prescribed medication in the US with more than 1 million prescriptions.[13]
Medical uses
[edit]Benign prostatic hyperplasia and prostate cancer
[edit]Dutasteride is used for treating BPH, colloquially known as an "enlarged prostate".[9][14] It is approved by the Food and Drug Administration (FDA) in the U.S. for this indication.[15] A 2010 Cochrane review found a 25–26% reduction in the risk of developing prostate cancer with 5α-reductase inhibitor chemoprevention.[16]
Scalp hair loss and excessive hair growth
[edit]Dutasteride is approved for the treatment of male androgenetic alopecia in South Korea and Japan at a dosage of 0.5 mg per day.[6][17] Several studies have found it to induce hair regrowth in men more rapidly and to a greater extent than even the highest approved dosage of finasteride.[6][18][19][20] The superior effectiveness of dutasteride relative to finasteride for this indication is because the inhibition of 5α-reductase and consequent reduction of dihydrotestosterone (DHT) production within the hair follicles is more complete with dutasteride. Dutasteride is also used off-label in the treatment of female pattern hair loss.[21][22]
Other 5α-reductase inhibitors such as finasteride (a type 2 inhibitor) have been used off-label to treat excessive hair growth in women with hirsutism.[3][23] Since dutasteride is an inhibitor of both type 1 and 2 5α-reductases, it could theoretically be a more effective therapy for hirsutism. However, dutasteride is not recommended for this indication due to a lack of supportive clinical evidence and a substantial risk of birth defects in female patients who inadvertently become pregnant.[23][24]
Transgender hormone therapy
[edit]Dutasteride is sometimes used as a component of hormone therapy for transgender women in combination with an estrogen and/or another antiandrogen such as spironolactone.[7] It may be useful for treating scalp hair loss or in those who have issues tolerating spironolactone.[7]
Available forms
[edit]Dutasteride is provided in the form of soft, oil-filled gelatin capsules containing 0.5 mg dutasteride each.[25]
Contraindications
[edit]Women who are or who may become pregnant should not handle the drug. Dutasteride can cause birth defects in male fetuses, specifically ambiguous genitalia and undermasculinization.[25][26] This is due to its antiandrogenic effects similar to what is seen in 5α-reductase deficiency.[26] For the same reason, women who are currently pregnant should never take dutasteride.[25] People taking dutasteride should not donate blood to prevent birth defects if a pregnant woman receives blood and should also not donate blood for at least 6 months after the cessation of treatment due to the drug's long elimination half-life.[25]
Children and people with known significant hypersensitivity (e.g., serious skin reactions, angioedema) to dutasteride should not take it.[25]
Adverse effects
[edit]Dutasteride has overall been found to be well tolerated in studies of both men and women, producing minimal side effects.[27] Adverse effects include headache and gastrointestinal discomfort.[27] Isolated reports of menstrual changes, acne, and dizziness also exist.[27] A small risk of sexual side effects has been documented in men taking the drug during the first few months of therapy.[27][28]
The FDA added a black-box warning to dutasteride in 2011 describing an increased risk of high-grade prostate cancer in those who take the drug.[29] No direct mechanistic link between 5α-reductase inhibitors and prostate cancer has been established.[30] This is not due to a direct link between dutasteride or other 5α-reductase inhibitors and cancer per se, but rather that those who take 5α-reductase inhibitors may have a decrease in prostate-specific antigen (PSA) levels, and therefore increases in PSA (which are an indicator of possible cancer) may be masked in those who take the drug.[31] This is thought to delay cancer diagnosis so that patients taking 5α-reductase inhibitors present with a higher-grade tumor at the time of diagnosis. The American Urological Association advises that increased risk for patients taking these drugs leads to higher prostate cancer-specific and all-cause mortality.[30] The AUA also advises that this affect can be alleviated with more frequent screening and lower PSA cutoffs for diagnostic biopsies in men taking dutasteride or other 5α-reductase inhibitors.[30] Dutasteride is known to reduce the growth and prevalence of benign prostate tumors.[32] A 2018 meta-analysis found no higher risk of breast cancer with 5α-reductase inhibitors.[33]
Sexual and mood side effects, such as erectile dysfunction,[34] loss of libido,[35] depression,[36] and reduced semen volume occur in as many as 4.8% of patients taking 5α-reductase inhibitors including dutasteride.[37][35] In affected men, semen volume is decreased an average of 30%,[38] with a smaller subgroup of patients also experiencing a decrease of sperm motility of 6-12%.[39][40] Sperm shape and function are unaffected and the impact on male fertility is unknown.[41] These negative effects reverse by 3–4 months after discontinuation of the drug.[41][40][30]
In a study of 6,729 men with benign prostatic hyperplasia (BPH, a condition where the prostate grows unassociated with cancer), 9% had erectile dysfunction (compared to 5.7% treated with a placebo), 3.3% experienced decreased sex drive (vs 1.6% of placebo), and 1.9% had enlarged breasts (vs 1% of placebo).[34][11] These effects were noted to resolve over time, with many fewer men reporting any adverse effects by the end of the 4-year study.[11][34] The rate of discontinuation of the drug due to adverse effects was less than 5%.[11]
A subset of men affected by sexual and mood side effects report persistent loss of libido,[34] depression,[27] and erectile dysfunction for several years after discontinuing treatment.[35] This remains a highly contested topic in the academic literature due to disagreements about whether the nocebo effect may play a role,[42][43][44] whether self-report questionnaires are reliable for this data,[30] and whether enough objective evidence exists to conclude these effects are persistent after discontinuation of the drug.[30][45][46] The Post-Finasteride Syndrome Foundation (PFSF) was created with a medical advisory board to study the topic (finasteride is a similar 5α-reductase inhibitor)[47] and lawsuits alleging harm from the drug are ongoing.[48] Concerns from the PFSF and other patient advocates led the FDA to add a black-box warning to Finasteride for possible risks of suicide in June 2022.[30][49] Some experts have questioned the basis of the black-box warning, given that it relies on anecdotal patient-reported outcomes rather than prospective trials.[30]
Overdose
[edit]No specific antidote for overdose of dutasteride is known, since the drug is extremely safe and well tolerated. Research studies show that even at 100 times the normal dose, dutasteride is not lethal.[50] Treatment of dutasteride overdose should be based on symptoms and should be with supportive therapies.[50] The long elimination half-life of dutasteride should be taken into consideration in the event of an overdose of the medication.[50] Dutasteride has been used in clinical studies at doses of up to 40 mg/day for a week (80 times the therapeutic dosage) and 5 mg/day for 6 months (10 times the therapeutic dosage) with no significant safety concerns or additional side effects.[50]
Current investigations
[edit]Dutasteride has been studied in combination with bicalutamide in the treatment of prostate cancer.[51][52][53]
Ongoing clinical trials are investigating whether dutasteride may be an effective treatment for premenstrual dysphoric disorder (PMDD), because dutasteride may inhibit the conversion of progesterone to allopregnanolone, a neurosteroid metabolite, which may be responsible for some of the debilitating symptoms of PMDD.[54][55]
Pharmacology
[edit]Pharmacodynamics
[edit]Dutasteride belongs to a class of drugs called 5α-reductase inhibitors, which block the action of the 5α-reductase enzymes that convert testosterone into DHT.[56] It inhibits all three forms of 5α-reductase, and can decrease DHT levels in the blood by up to 98%.[1][57][58] Specifically it is a competitive, mechanism-based (irreversible) inhibitor of all three isoforms of 5α-reductase, types I, II, and III (IC50 values are 3.9 nM for type I and 1.8 nM for type II).[1][57][59][60] This is in contrast to finasteride, which is similarly an irreversible inhibitor of 5α-reductase but only inhibits the type II and III isoenzymes.[60][61][57] As a result of this difference, dutasteride is able to achieve a reduction in circulating DHT levels of up to 98%, whereas finasteride is able to achieve a reduction of only 65 to 70%.[58][2][56][62] In spite of the differential reduction in circulating DHT levels, the two drugs decrease levels of DHT to a similar extent of approximately 85 to 90% in the prostate gland,[62] where the type II isoform predominates.[59]
Since 5α-reductases degrade testosterone to DHT, the inhibition of these enzymes could theoretically cause an increase in testosterone. A 2018 review found that initiation of 5α-reductase inhibitors did not result in a consistent increase in testosterone levels.[63] Among the studies analyzed, there was no statistically significant change in testosterone levels from 5α-reductase inhibitors overall, though men with lower baseline testosterone levels did show an increase.[63]
In addition to inhibition of DHT production, 5α-reductase inhibitors such as dutasteride are also neurosteroidogenesis inhibitors, preventing the 5α-reductase-mediated biosynthesis of various neurosteroids, including allopregnanolone (from progesterone), THDOC (from deoxycorticosterone), and 3α-androstanediol (from testosterone).[37] These neurosteroids are potent positive allosteric modulators of the GABAA receptor and have shown antidepressant, anxiolytic, and pro-sexual effects in animal research.[37][64][65] For this reason, decreased neurosteroid production is one hypothesized mechanism for sexual dysfunction and depression associated with 5α-reductase inhibitors such as dutasteride.[37]
Pharmacokinetics
[edit]The oral bioavailability of dutasteride is about 60%.[1] Consumption with food does not adversely affect its absorption.[1] Peak plasma levels occur 2 to 3 hours after administration.[1] Dutasteride is present in semen at levels up to 3 ng/ml, with no significant effects on DHT levels of sexual partners.[1] The drug is extensively metabolized in the liver by CYP3A4.[1] It has three major metabolites: 6'-hydroxydutasteride, 4'-hydroxydutasteride, and 1,2-dihydrodutasteride. The former two are formed by CYP3A4, while the latter is not.[1] All three metabolites are active; 6'-hydroxydutasteride has similar 5α-reductase inhibitor potency as dutasteride, while the other two are less potent.[1] Dutasteride has an extremely long terminal or elimination half-life of about 4 to 5 weeks.[2][3] Its elimination half-life is increased in the elderly (170 hours for men aged 20–49 years, 300 hours for men aged >70 years).[1] No dosage adjustment is necessary in the elderly nor in patients with renal impairment.[1] Because of its long elimination half-life, dutasteride requires 5 to 6 months to reach steady-state concentrations.[59] It also remains in the body for a long time after discontinuation and can be detected up to 4 to 6 months.[1][2] In contrast to dutasteride, finasteride has a short terminal half-life of only 5 to 8 hours.[3][1] Dutasteride is eliminated mainly in the feces (40%) as metabolites.[1] A smaller portion (5%) is eliminated unchanged in the urine.[1]
Chemistry
[edit]Dutasteride, also known as N-[2,5-bis(trifluoromethyl)phenyl]-3-oxo-4-aza-5α-androst-1-ene-17β-carboxamide, is a synthetic androstane steroid and a 4-azasteroid.[66][67] It is an analogue of finasteride in which the tert-butyl amide moiety has been replaced with a 2,5-bis(trifluoromethyl)phenyl group.[67]
History
[edit]Dutasteride was patented in 1996 and was first described in the scientific literature in 1997.[68][69] It was approved by the FDA for the treatment of BPH in November 2001, and was introduced on the United States market the following year under the brand name Avodart.[69] Dutasteride has subsequently been introduced in many other countries, including throughout Europe and South America.[69] The patent protection of dutasteride expired in November 2015, so the drug has since become available in the United States in a variety of low-cost generic formulations.[68]
It was approved for the treatment of scalp hair loss in South Korea in 2009 and in Japan in 2015.[70] It has not been approved for this indication in the United States,[6][17] though it is often used off-label.[21]
Society and culture
[edit]
Generic names
[edit]Dutasteride is the generic name of the drug Avodart and its international nonproprietary name, United States Adopted Name, British Approved Name, and Japanese Accepted Name.[71]
Brand names
[edit]Dutasteride is sold primarily under the brand name Avodart, but also in combination with tamsulosin under the brand names Combodart, Duodart, and Jalyn.[71] Dutasteride is also available in India in combination with alfuzosin under the brand names Alfusin-D and Dutalfa.[71]
Availability
[edit]Dutasteride is available widely throughout the world, including in the United States, Canada, the United Kingdom, Ireland, Europe, Australia, South Africa, Latin America, Asia, and elsewhere.[71] It is available as a generic medication in many countries, including the United States.[68]
References
[edit]- ^ a b c d e f g h i j k l m n o p q r s t u v w x Lemke TL, Williams DA (2008). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 1286–1287. ISBN 978-0-7817-6879-5. Archived from the original on 10 January 2023. Retrieved 6 December 2017.
- ^ a b c d Burchum J, Rosenthal L (2 December 2014). Lehne's Pharmacology for Nursing Care. Elsevier Health Sciences. pp. 803–. ISBN 978-0-323-34026-7. Archived from the original on 12 January 2023. Retrieved 27 October 2016.
- ^ a b c d e Blume-Peytavi U, Whiting DA, Trüeb RM (26 June 2008). Hair Growth and Disorders. Springer Science & Business Media. pp. 182, 369. ISBN 978-3-540-46911-7. Archived from the original on 10 January 2023. Retrieved 10 December 2016.
- ^ https://pubs.rsc.org/en/content/getauthorversionpdf/C5CE00036J
- ^ a b c British National Formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 769. ISBN 9780857113382.
- ^ a b c d Shapiro J, Otberg N (17 April 2015). Hair Loss and Restoration, Second Edition. CRC Press. pp. 39–. ISBN 978-1-4822-3199-1. Archived from the original on 12 January 2023. Retrieved 27 October 2016.
- ^ a b c Wesp LM, Deutsch MB (March 2017). "Hormonal and Surgical Treatment Options for Transgender Women and Transfeminine Spectrum Persons". The Psychiatric Clinics of North America. 40 (1): 99–111. doi:10.1016/j.psc.2016.10.006. PMID 28159148.
- ^ a b c d "Dutasteride Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 4 July 2019. Retrieved 18 March 2019.
- ^ a b Wu C, Kapoor A (July 2013). "Dutasteride for the treatment of benign prostatic hyperplasia". Expert Opinion on Pharmacotherapy. 14 (10): 1399–1408. doi:10.1517/14656566.2013.797965. PMID 23750593. S2CID 25041466.
- ^ Fertig RM, Gamret AC, Darwin E, Gaudi S (November 2017). "Sexual side effects of 5-α-reductase inhibitors finasteride and dutasteride: A comprehensive review". Dermatology Online Journal. 23 (11). doi:10.5070/D32311037240. PMID 29447628.
- ^ a b c d Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, Montorsi F, et al. (April 2010). "Effect of dutasteride on the risk of prostate cancer". The New England Journal of Medicine. 362 (13): 1192–1202. doi:10.1056/NEJMoa0908127. PMID 20357281.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 483. ISBN 9783527607495. Archived from the original on 10 January 2023. Retrieved 19 September 2020.
- ^ "Dutasteride - Drug Usage Statistics". ClinCalc. Archived from the original on 6 February 2020. Retrieved 7 October 2022.
- ^ Slater S, Dumas C, Bubley G (March 2012). "Dutasteride for the treatment of prostate-related conditions". Expert Opinion on Drug Safety. 11 (2): 325–330. doi:10.1517/14740338.2012.658040. PMID 22316171. S2CID 207487490.
- ^ "Search Results for "DUTASTERIDE"". Drugs@FDA: FDA Approved Drug Products. Archived from the original on 29 August 2021. Retrieved 29 October 2016.
- ^ Wilt TJ, Macdonald R, Hagerty K, Schellhammer P, Tacklind J, Somerfield MR, et al. (November 2010). "5-α-Reductase inhibitors for prostate cancer chemoprevention: an updated Cochrane systematic review". BJU International. 106 (10): 1444–1451. doi:10.1111/j.1464-410X.2010.09714.x. PMID 20977593. S2CID 22178061.
- ^ a b Choi GS, Kim JH, Oh SY, Park JM, Hong JS, Lee YS, et al. (August 2016). "Safety and Tolerability of the Dual 5-Alpha Reductase Inhibitor Dutasteride in the Treatment of Androgenetic Alopecia". Annals of Dermatology. 28 (4): 444–450. doi:10.5021/ad.2016.28.4.444. PMC 4969473. PMID 27489426.
- ^ Dhurat R, Sharma A, Rudnicka L, Kroumpouzos G, Kassir M, Galadari H, et al. (May 2020). "5-Alpha reductase inhibitors in androgenetic alopecia: Shifting paradigms, current concepts, comparative efficacy, and safety". Dermatologic Therapy. 33 (3): e13379. doi:10.1111/dth.13379. PMID 32279398. S2CID 215748750.
- ^ Zhou Z, Song S, Gao Z, Wu J, Ma J, Cui Y (2019). "The efficacy and safety of dutasteride compared with finasteride in treating men with androgenetic alopecia: a systematic review and meta-analysis". Clinical Interventions in Aging. 14: 399–406. doi:10.2147/CIA.S192435. PMC 6388756. PMID 30863034.
- ^ Olsen EA, Hordinsky M, Whiting D, Stough D, Hobbs S, Ellis ML, et al. (December 2006). "The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride". Journal of the American Academy of Dermatology. 55 (6): 1014–1023. doi:10.1016/j.jaad.2006.05.007. PMID 17110217.
- ^ a b Nusbaum AG, Rose PT, Nusbaum BP (August 2013). "Nonsurgical therapy for hair loss". Facial Plastic Surgery Clinics of North America. 21 (3): 335–342. doi:10.1016/j.fsc.2013.04.003. PMID 24017975.
- ^ Carmina E, Azziz R, Bergfeld W, Escobar-Morreale HF, Futterweit W, Huddleston H, et al. (July 2019). "Female Pattern Hair Loss and Androgen Excess: A Report From the Multidisciplinary Androgen Excess and PCOS Committee". The Journal of Clinical Endocrinology and Metabolism. 104 (7): 2875–2891. doi:10.1210/jc.2018-02548. PMID 30785992.
- ^ a b Martin KA, Chang RJ, Ehrmann DA, Ibanez L, Lobo RA, Rosenfield RL, et al. (April 2008). "Evaluation and treatment of hirsutism in premenopausal women: an endocrine society clinical practice guideline". The Journal of Clinical Endocrinology and Metabolism. 93 (4): 1105–1120. doi:10.1210/jc.2018-00241. PMID 18252793.
- ^ Lebwohl MG, Heymann WR, Berth-Jones J, Coulson I (19 September 2013). Treatment of Skin Disease: Comprehensive Therapeutic Strategies. Elsevier Health Sciences. pp. 327–. ISBN 978-0-7020-5236-1. Archived from the original on 12 January 2023. Retrieved 10 December 2016.
- ^ a b c d e "AVODART (dutasteride) Soft Gelatin Capsules Prescribing information" (PDF). GlaxoSmithKline. U.S. Food and Drug Administration. June 2011. Archived (PDF) from the original on 7 March 2013. Retrieved 15 September 2013.
- ^ a b McVary KT, Welliver C (12 August 2016). Treatment of Lower Urinary Tract Symptoms and Benign Prostatic Hyperplasia: Current methods, outcomes, and controversies, An Issue of Urologic Clinics of North America, E-Book. Elsevier Health Sciences. pp. 396–. ISBN 978-0-323-45994-5. Archived from the original on 12 January 2023. Retrieved 10 December 2017.
- ^ a b c d e Hirshburg JM, Kelsey PA, Therrien CA, Gavino AC, Reichenberg JS (July 2016). "Adverse Effects and Safety of 5-alpha Reductase Inhibitors (Finasteride, Dutasteride): A Systematic Review". The Journal of Clinical and Aesthetic Dermatology. 9 (7): 56–62. PMC 5023004. PMID 27672412.
- ^ Trost L, Saitz TR, Hellstrom WJ (May 2013). "Side Effects of 5-Alpha Reductase Inhibitors: A Comprehensive Review". Sexual Medicine Reviews. 1 (1): 24–41. doi:10.1002/smrj.3. PMID 27784557.
- ^ "FDA Drug Safety Communication: 5-alpha reductase inhibitors (5-ARIs) may increase the risk of a more serious form of prostate cancer". U.S. Food and Drug Administration. 18 June 2019. Archived from the original on 9 March 2021. Retrieved 9 March 2021.
- ^ a b c d e f g h Lerner LB, McVary KT, Barry MJ, Bixler BR, Dahm P, Das AK, et al. (October 2021). "Management of Lower Urinary Tract Symptoms Attributed to Benign Prostatic Hyperplasia: AUA GUIDELINE PART I-Initial Work-up and Medical Management". The Journal of Urology. 206 (4): 806–817. doi:10.1097/JU.0000000000002183. PMID 34384237. S2CID 236999299.
- ^ Sarkar RR, Parsons JK, Bryant AK, Ryan ST, Kader AK, McKay RR, et al. (June 2019). "Association of Treatment With 5α-Reductase Inhibitors With Time to Diagnosis and Mortality in Prostate Cancer". JAMA Internal Medicine. 179 (6): 812–819. doi:10.1001/jamainternmed.2019.0280. PMC 6503564. PMID 31058923.
- ^ Walsh PC (April 2010). "Chemoprevention of prostate cancer". The New England Journal of Medicine. 362 (13): 1237–1238. doi:10.1056/NEJMe1001045. PMID 20357287.
- ^ Wang J, Zhao S, Luo L, Li E, Li X, Zhao Z (2018). "5-alpha Reductase Inhibitors and risk of male breast cancer: a systematic review and meta-analysis". International Braz J Urol. 44 (5): 865–873. doi:10.1590/S1677-5538.IBJU.2017.0531. PMC 6237523. PMID 29697934.
- ^ a b c d Fertig R, Shapiro J, Bergfeld W, Tosti A (January 2017). "Investigation of the Plausibility of 5-Alpha-Reductase Inhibitor Syndrome". Skin Appendage Disorders. 2 (3–4): 120–129. doi:10.1159/000450617. PMC 5264352. PMID 28232919.
- ^ a b c Traish AM, Melcangi RC, Bortolato M, Garcia-Segura LM, Zitzmann M (September 2015). "Adverse effects of 5α-reductase inhibitors: What do we know, don't know, and need to know?". Reviews in Endocrine & Metabolic Disorders. 16 (3): 177–198. doi:10.1007/s11154-015-9319-y. PMID 26296373. S2CID 25002351.
- ^ Traish AM, Hassani J, Guay AT, Zitzmann M, Hansen ML (March 2011). "Adverse side effects of 5α-reductase inhibitors therapy: persistent diminished libido and erectile dysfunction and depression in a subset of patients". The Journal of Sexual Medicine. 8 (3): 872–884. doi:10.1111/j.1743-6109.2010.02157.x. PMID 21176115.
- ^ a b c d Traish AM, Mulgaonkar A, Giordano N (June 2014). "The dark side of 5α-reductase inhibitors' therapy: sexual dysfunction, high Gleason grade prostate cancer and depression". Korean Journal of Urology. 55 (6): 367–379. doi:10.4111/kju.2014.55.6.367. PMC 4064044. PMID 24955220.
- ^ Samplaski MK, Lo K, Grober E, Jarvi K (December 2013). "Finasteride use in the male infertility population: effects on semen and hormone parameters". Fertility and Sterility. 100 (6): 1542–1546. doi:10.1016/j.fertnstert.2013.07.2000. PMID 24012200.
- ^ Amory JK, Wang C, Swerdloff RS, Anawalt BD, Matsumoto AM, Bremner WJ, et al. (May 2007). "The effect of 5alpha-reductase inhibition with dutasteride and finasteride on semen parameters and serum hormones in healthy men". The Journal of Clinical Endocrinology and Metabolism. 92 (5): 1659–1665. doi:10.1210/jc.2006-2203. PMID 17299062.
- ^ a b Millsop JW, Heller MM, Eliason MJ, Murase JE (July 2013). "Dermatological medication effects on male fertility". Dermatologic Therapy. 26 (4): 337–346. doi:10.1111/dth.12069. PMID 23914891. S2CID 9087715.
- ^ a b Semet M, Paci M, Saïas-Magnan J, Metzler-Guillemain C, Boissier R, Lejeune H, et al. (July 2017). "The impact of drugs on male fertility: a review". Andrology. 5 (4): 640–663. doi:10.1111/andr.12366. PMID 28622464. S2CID 37989045.
- ^ Than JK, Rodriguez K, Khera M (24 July 2018). "Post-finasteride Syndrome: A Review of Current Literature". Current Sexual Health Reports. 10 (3): 152–157. doi:10.1007/s11930-018-0163-4. eISSN 1548-3592. ISSN 1548-3584. S2CID 81968700.
- ^ Saengmearnuparp T, Lojanapiwat B, Chattipakorn N, Chattipakorn S (November 2021). "The connection of 5-alpha reductase inhibitors to the development of depression". Biomedicine & Pharmacotherapy. 143: 112100. doi:10.1016/j.biopha.2021.112100. PMID 34479019.
- ^ Coskuner ER, Ozkan B, Culha MG (April 2019). "Sexual Problems of Men With Androgenic Alopecia Treated With 5-Alpha Reductase Inhibitors". Sexual Medicine Reviews. 7 (2): 277–282. doi:10.1016/j.sxmr.2018.07.003. PMID 30301703. S2CID 52946784.
- ^ Traish AM (January 2020). "Post-finasteride syndrome: a surmountable challenge for clinicians". Fertility and Sterility. 113 (1): 21–50. doi:10.1016/j.fertnstert.2019.11.030. PMID 32033719. S2CID 211064052.
- ^ Liu L, Zhao S, Li F, Li E, Kang R, Luo L, et al. (September 2016). "Effect of 5α-Reductase Inhibitors on Sexual Function: A Meta-Analysis and Systematic Review of Randomized Controlled Trials". The Journal of Sexual Medicine. 13 (9): 1297–1310. doi:10.1016/j.jsxm.2016.07.006. PMID 27475241.
- ^ "The Post-Finasteride Syndrome Foundation – Dedicated to supporting research and finding treatments for PFS patients worldwide". Archived from the original on 21 December 2021. Retrieved 24 December 2021.
- ^ Pierson B (8 September 2021). "Group sues to have hair-loss drug Propecia pulled from market". Reuters. Archived from the original on 24 December 2021. Retrieved 24 December 2021.
- ^ Levine D (10 June 2022). "FDA requires disclosure of suicide risk for anti-baldness drug". Reuters. Archived from the original on 12 November 2022. Retrieved 12 November 2022.
- ^ a b c d "AVODART (dutasteride) Soft Gelatin Capsules Prescribing information" (PDF). GlaxoSmithKline. U.S. Food and Drug Administration. Archived (PDF) from the original on 3 April 2021. Retrieved 10 January 2020.
- ^ Chu FM, Sartor O, Gomella L, Rudo T, Somerville MC, Hereghty B, et al. (August 2015). "A randomised, double-blind study comparing the addition of bicalutamide with or without dutasteride to GnRH analogue therapy in men with non-metastatic castrate-resistant prostate cancer". European Journal of Cancer. 51 (12): 1555–1569. doi:10.1016/j.ejca.2015.04.028. PMID 26048455.
- ^ Gaudet M, Vigneault É, Foster W, Meyer F, Martin AG (January 2016). "Randomized non-inferiority trial of Bicalutamide and Dutasteride versus LHRH agonists for prostate volume reduction prior to I-125 permanent implant brachytherapy for prostate cancer". Radiotherapy and Oncology. 118 (1): 141–147. doi:10.1016/j.radonc.2015.11.022. PMID 26702991.
- ^ Dijkstra S, Witjes WP, Roos EP, Vijverberg PL, Geboers AD, Bruins JL, et al. (2016). "The AVOCAT study: Bicalutamide monotherapy versus combined bicalutamide plus dutasteride therapy for patients with locally advanced or metastatic carcinoma of the prostate-a long-term follow-up comparison and quality of life analysis". SpringerPlus. 5: 653. doi:10.1186/s40064-016-2280-8. PMC 4870485. PMID 27330919.
- ^ Pearlstein T (April 2016). "Treatment of Premenstrual Dysphoric Disorder: Therapeutic Challenges". Expert Review of Clinical Pharmacology. 9 (4): 493–496. doi:10.1586/17512433.2016.1142371. PMID 26766596.
A recent study with a 5α-reductase inhibitor dutasteride, that blocks the conversion of progesterone to ALLO, reported that dutasteride 2.5 mg daily decreased several premenstrual symptoms
- ^ Naguy A, El-Sheshai A, Thiguti SH, Alamiri B (June 2022). "Psychopharmacotherapy of Premenstrual Dysphoric Disorder-New Vistas". Psychopharmacology Bulletin. 52 (3): 81–83. PMC 9235312. PMID 35815174.
Capitalizing on this premise, agents in the pipeline for PMDD including dutasteride, ulipristal acetate, and sepranolone are promising. Dutasteride, FDA-approved for benign prostatic hyperplasia, is a 5-α reductase inhibitor; the latter catalyzes the rate-limiting step in metabolism of progesterone to allopregnanolone...Two double-blind RCTs, cross-over trials, support use of dutasteride where high-dose (2.5 mg/d) outperforms placebo.
- ^ a b Bostwick DG, Cheng L (24 January 2014). Urologic Surgical Pathology. Elsevier Health Sciences. pp. 492–. ISBN 978-0-323-08619-6.
- ^ a b c Yamana K, Labrie F, Luu-The V (August 2010). "Human type 3 5α-reductase is expressed in peripheral tissues at higher levels than types 1 and 2 and its activity is potently inhibited by finasteride and dutasteride". Hormone Molecular Biology and Clinical Investigation. 2 (3): 293–299. doi:10.1515/hmbci.2010.035. PMID 25961201. S2CID 28841145.
- ^ a b Bradbury R (30 January 2007). Cancer. Springer Science & Business Media. pp. 49–. ISBN 978-3-540-33120-9.
- ^ a b c Keam SJ, Scott LJ (2008). "Dutasteride: a review of its use in the management of prostate disorders". Drugs. 68 (4): 463–485. doi:10.2165/00003495-200868040-00008. PMID 18318566. S2CID 242987808.
- ^ a b Gisleskog PO, Hermann D, Hammarlund-Udenaes M, Karlsson MO (December 1998). "A model for the turnover of dihydrotestosterone in the presence of the irreversible 5 alpha-reductase inhibitors GI198745 and finasteride". Clinical Pharmacology and Therapeutics. 64 (6): 636–647. doi:10.1016/S0009-9236(98)90054-6. PMID 9871428. S2CID 42901328.
- ^ Keserü G, Swinney DC (28 July 2015). Thermodynamics and Kinetics of Drug Binding. Wiley. pp. 165–. ISBN 978-3-527-67304-9.
- ^ a b Heesakkers J, Chapple C, De Ridder D, Farag F (24 February 2016). Practical Functional Urology. Springer. pp. 280–. ISBN 978-3-319-25430-2.
- ^ a b Traish AM, Krakowsky Y, Doros G, Morgentaler A (January 2019). "Do 5α-Reductase Inhibitors Raise Circulating Serum Testosterone Levels? A Comprehensive Review and Meta-Analysis to Explaining Paradoxical Results". Sexual Medicine Reviews. 7 (1): 95–114. doi:10.1016/j.sxmr.2018.06.002. PMID 30098986. S2CID 51968365.
- ^ Weizman A (1 February 2008). Neuroactive Steroids in Brain Function, Behavior and Neuropsychiatric Disorders: Novel Strategies for Research and Treatment. Springer Science & Business Media. ISBN 978-1-4020-6854-6.
- ^ Tvrdeić A, Poljak L (2016). "Neurosteroids, GABAA receptors and neurosteroid based drugs: are we witnessing the dawn of the new psychiatric drugs?". Endocrine Oncology and Metabolism. 2 (1): 60–71. doi:10.21040/eom/2016.2.7 (inactive 1 November 2024).
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ Lemke TL, Williams DA (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 1381–. ISBN 978-1-60913-345-0.
- ^ a b Ravina E (11 January 2011). The Evolution of Drug Discovery: From Traditional Medicines to Modern Drugs. John Wiley & Sons. pp. 183–. ISBN 978-3-527-32669-3.
- ^ a b c "Generic Avodart Availability". Drugs.com. Archived from the original on 20 December 2016. Retrieved 10 December 2016.
- ^ a b c Llewellyn W (2011). Anabolics. Molecular Nutrition Llc. pp. 968–, 971–. ISBN 978-0-9828280-1-4. Archived from the original on 12 January 2023. Retrieved 11 December 2017.
- ^ MacDonald G (3 December 2015). "GSK Japan delays alopecia drug launch after Catalent manufacturing halt". Archived from the original on 1 October 2016. Retrieved 14 June 2017.
- ^ a b c d "Dutasteride". Drugs.com. Archived from the original on 11 December 2017. Retrieved 11 December 2017.
Further reading
[edit]- Frye SV (2006). "Discovery and clinical development of dutasteride, a potent dual 5alpha-reductase inhibitor". Current Topics in Medicinal Chemistry. 6 (5): 405–421. doi:10.2174/156802606776743101. PMID 16719800.
