Acoramidis
 | |
| Clinical data | |
|---|---|
| Pronunciation | ə-corAM-i-dis |
| Trade names | Attruby |
| Other names | AG10 |
| AHFS/Drugs.com | Attruby |
| License data |
|
| Routes of administration | By mouth |
| Drug class | Amyloidogenesis suppressant |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
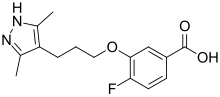
| Formula | C15H17FN2O3 |
| Molar mass | 292.310 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Acoramidis, sold under the brand name Attruby, is a medication used for the treatment of cardiomyopathy.[1] It is a near-complete (>90%) transthyretin stabilizer, developed to mimic the protective properties of the naturally-occurring T119M mutation,[2][3] to treat transthyretin amyloid cardiomyopathy. It is taken by mouth.[1]
The most common adverse reactions include diarrhea and upper abdominal pain.[4]
Acoramidis was approved for medical use in the United States in November 2024.[4][5][6]
Medical uses
[edit]Acoramidis is indicated for the treatment of the cardiomyopathy of wild-type or variant transthyretin-mediated amyloidosis (ATTR-CM) in adults to reduce cardiovascular death and cardiovascular-related hospitalization.[1][4][7]
ATTR-CM is a rare and serious disease that affects the heart muscle.[4] In people with ATTR-CM, there is a build-up of protein deposits in the heart, causing the walls of the heart to become stiff, and making the left ventricle unable to properly relax and fill with blood (called cardiomyopathy).[4] As the condition progresses, the heart can become unable to pump blood out adequately, causing heart failure.[4] There are two types of ATTR-CM, hereditary ATTR-CM (hATTR-CM) and wild-type ATTR-CM (wATTR-CM).[4] In hATTR-CM, which can run in families, there's a variant in the transthyretin gene, which results in protein deposits in the heart. In wATTR-CM, there is no variant in the transthyretin gene.[4]
Side effects
[edit]The most common side effects are diarrhea and abdominal pain.[8]
History
[edit]The efficacy and safety of acoramidis were evaluated in a multicenter, international, randomized, double-blind, placebo-controlled study in 611 adult participants with wild-type or hereditary (variant) ATTR-CM (NCT03860935).[4]
Clinical trials
[edit]Phase I data indicated acoramidis achieved near-complete (>90%) TTR stabilization across the entire dosing interval at steady state.[9]
Phase II and the Open-Label Extension (OLE) data indicated after a median of 38 months, long-term treatment with acoramidis was generally well tolerated and resulted in a median decline in NT-proBNP levels, normalization of serum TTR, and sustained stabilization of TTR in individuals with ATTR-CM. [10]
Phase III data from ATTRibute-CM indicated acoramidis resulted in a significantly better four-step primary hierarchical outcome containing components of mortality, morbidity, and function than placebo at 30 months in participants with ATTR-CM. Adverse events were similar in the two groups.[11]
Other analyses from ATTRibute-CM indicated a 50% reduction in cumulative cardiovascular hospitalizations (CVH), a 42% reduction in all-cause mortality (ACM) and recurrent CVH, and a 3-month time-to-separation of the Kaplan Meier curves for ACM or CVH. No other treatment has demonstrated this degree of treatment effect this quickly in participants with ATTR-CM.[12][13][14]
In vitro data indicated acoramidis exhibits near-complete (>90%) TTR stabilization at therapeutic trough concentrations, and its TTR stabilization exceeds that of tafamidis' across a range of destabilizing TTR mutations.[15]
Society and culture
[edit]Legal status
[edit]Acoramidis was approved for medical use in the United States in November 2024.[4][5][16] The approval was granted to BridgeBio Pharma.[7]
In December 2024, the Committee for Medicinal Products for Human Use of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Beyonttra, intended for the treatment of transthyretin amyloidosis in adults with cardiomyopathy.[17] The applicant for this medicinal product is BridgeBio Europe B.V.[17]
Acoramidis was designated an orphan medicine by the EMA.[17]
Names
[edit]During development, acoramidis was known as AG10 (the Alhamadsheh-Graef molecule 10).[18]
Acoramidis is the international nonproprietary name.[19]
References
[edit]- ^ a b c d "Attruby- acoramidis hydrochloride tablet, film coated". DailyMed. 26 November 2024. Retrieved 28 November 2024.
- ^ Penchala SC, Connelly S, Wang Y, Park MS, Zhao L, Baranczak A, et al. (June 2013). "AG10 inhibits amyloidogenesis and cellular toxicity of the familial amyloid cardiomyopathy-associated V122I transthyretin". Proceedings of the National Academy of Sciences of the United States of America. 110 (24): 9992–9997. doi:10.1073/pnas.1300761110. PMC 3683741. PMID 23716704.
- ^ Miller M, Pal A, Albusairi W, Joo H, Pappas B, Haque Tuhin MT, et al. (September 2018). "Enthalpy-Driven Stabilization of Transthyretin by AG10 Mimics a Naturally Occurring Genetic Variant That Protects from Transthyretin Amyloidosis". Journal of Medicinal Chemistry. 61 (17): 7862–7876. doi:10.1021/acs.jmedchem.8b00817. PMC 6276790. PMID 30133284.
- ^ a b c d e f g h i j "FDA approves drug for heart disorder caused by transthyretin-mediated". U.S. Food and Drug Administration. 1 October 2024. Retrieved 27 November 2024.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b "Novel Drug Approvals for 2024". U.S. Food and Drug Administration (FDA). 1 October 2024. Retrieved 20 December 2024.
- ^ "FDA approves BridgeBio Pharma's Attruby to treat rare heart disease ATTR-CM". PMLiVE. 25 November 2024. Retrieved 25 November 2024.
- ^ a b LeMieux J (25 November 2024). "Bridgebio's Attruby, to Treat Heart Condition ATTR-CM, Receives FDA Approval". Genetic Engineering and Biotechnology News. Retrieved 25 November 2024.
- ^ "FDA approves BridgeBio's Attruby for ATTR-CM treatment". Pharmaceutical Technology. 25 November 2024. Retrieved 25 November 2024.
- ^ Fox JC, Hellawell JL, Rao S, O'Reilly T, Lumpkin R, Jernelius J, et al. (January 2020). "First-in-Human Study of AG10, a Novel, Oral, Specific, Selective, and Potent Transthyretin Stabilizer for the Treatment of Transthyretin Amyloidosis: A Phase 1 Safety, Tolerability, Pharmacokinetic, and Pharmacodynamic Study in Healthy Adult Volunteers". Clinical Pharmacology in Drug Development. 9 (1): 115–129. doi:10.1002/cpdd.700. PMC 7003869. PMID 31172685.
{{cite journal}}: CS1 maint: overridden setting (link) - ^ Masri A, Aras M, Falk RH, Grogan M, Jacoby D, Judge DP, et al. (March 2022). "Long-Term Safety and Tolerability of Acoramidis (Ag10) in Symptomatic Transthyretin Amyloid Cardiomyopathy: Updated Analysis from an Ongoing Phase 2 Open-Label Extension Study". Journal of the American College of Cardiology. 79 (9): 227. doi:10.1016/S0735-1097(22)01218-9.
- ^ Gillmore JD, Judge DP, Cappelli F, Fontana M, Garcia-Pavia P, Gibbs S, et al. (January 2024). "Efficacy and Safety of Acoramidis in Transthyretin Amyloid Cardiomyopathy". The New England Journal of Medicine. 390 (2): 132–142. doi:10.1056/NEJMoa2305434. PMID 38197816.
{{cite journal}}: CS1 maint: overridden setting (link) - ^ "Program Planner". www.abstractsonline.com. Archived from the original on 6 February 2021. Retrieved 19 October 2024.
- ^ Alexander K, Judge D, Cappelli F, Fontana M, Garcia-Pavia P, Grogan M, et al. (6 May 2024). Acoramidis Achieves Early Reduction in Cardiovascular Death or Hospitalization in Transthyretin Amyloid Cardiomyopathy (ATTR-CM): Results from the ATTRibute-CM Clinical Trial OC7 (#281) (Report). doi:10.26226/m.65f9bf8ae6f73964e1d4f069.
- ^ "BridgeBio Shares Recurrent Event Analysis of ATTRibute-CM, Demonstrating a 42% Reduction by Acoramidis on the Composite Endpoint of All-Cause Mortality and Recurrent Cardiovascular-related Hospitalization Events". HFSA. Retrieved 19 October 2024.
- ^ Ji A, Wong P, Judge DP, Graef IA, Fox J, Sinha U (November 2023). "Acoramidis produces near-complete TTR stabilization in blood samples from patients with variant transthyretin amyloidosis that is greater than that achieved with tafamidis". European Heart Journal. 44 (Supplement_2). doi:10.1093/eurheartj/ehad655.989. ISSN 0195-668X.
- ^ "Attruby (acoramidis), a Near Complete TTR Stabilizer (≥90%), approved by FDA to Reduce Cardiovascular Death and Cardiovascular-related Hospitalization in ATTR-CM Patients" (Press release). BridgeBio Pharma. 23 November 2024. Archived from the original on 25 November 2024. Retrieved 28 November 2024 – via GlobeNewswire.
- ^ a b c "Beyonttra EPAR". European Medicines Agency (EMA). 12 December 2024. Retrieved 15 December 2024. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ "FDA approves Stanford Medicine-developed drug that treats rare heart disease". Stanford. 27 November 2024. Retrieved 29 November 2024.
- ^ World Health Organization (2024). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 83". WHO Drug Information. 38 (1). hdl:10665/378096.
Further reading
[edit]- Penchala SC, Connelly S, Wang Y, Park MS, Zhao L, Baranczak A, et al. (June 2013). "AG10 inhibits amyloidogenesis and cellular toxicity of the familial amyloid cardiomyopathy-associated V122I transthyretin". Proceedings of the National Academy of Sciences of the United States of America. 110 (24): 9992–9997. doi:10.1073/pnas.1300761110. PMC 3683741. PMID 23716704.
{{cite journal}}: CS1 maint: overridden setting (link)
External links
[edit]- Clinical trial number NCT03860935 for "Efficacy and Safety of AG10 in Subjects With Transthyretin Amyloid Cardiomyopathy (ATTRibute-CM)" at ClinicalTrials.gov
