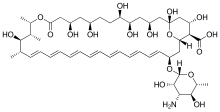
Amphotericin B
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Fungizone, Mysteclin-F, AmBisome and other |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682643 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Intravenous infusion |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 100% (IV) |
| Metabolism | kidney |
| Elimination half-life |
|
| Excretion |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.014.311 |
| Chemical and physical data | |
| Formula | C47H73NO17 |
| Molar mass | 924.091 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 170 °C (338 °F) |
| |
| |
| (verify) | |
Amphotericin B is an antifungal medication used for serious fungal infections and leishmaniasis.[3] The fungal infections it is used to treat include mucormycosis, aspergillosis, blastomycosis, candidiasis, coccidioidomycosis, and cryptococcosis.[4] For certain infections it is given with flucytosine.[5] It is typically given intravenously (injection into a vein).[4]
Common side effects include a reaction with fever, chills, and headaches soon after the medication is given, as well as kidney problems.[4] Allergic symptoms including anaphylaxis may occur.[4] Other serious side effects include low blood potassium and myocarditis (inflammation of the heart).[3] It appears to be relatively safe in pregnancy.[4] There is a lipid formulation that has a lower risk of side effects.[4] It is in the polyene class of medications and works in part by interfering with the cell membrane of the fungus.[3][4]
Amphotericin B was isolated from Streptomyces nodosus in 1955 at the Squibb Institute for Medical Research from cultures isolated from the streptomycete obtained from the river bed of Orinoco in that region of Venezuela[6] and came into medical use in 1958.[7][8] It is on the World Health Organization's List of Essential Medicines.[9] It is available as a generic medication.[4][10]
Medical uses
[edit]Antifungal
[edit]One of the main uses of amphotericin B is treating a wide range of systemic fungal infections. Due to its extensive side effects, it is often reserved for severe infections in critically ill, or immunocompromised patients. It is considered first line therapy for invasive mucormycosis infections, cryptococcal meningitis, and certain aspergillus and candidal infections.[11][12] It has been a highly effective drug for over fifty years in large part because it has a low incidence of drug resistance in the pathogens it treats. This is because amphotericin B resistance requires sacrifices on the part of the pathogen that make it susceptible to the host environment, and too weak to cause infection.[13]
Antiprotozoal
[edit]Amphotericin B is used for life-threatening protozoan infections such as visceral leishmaniasis[14] and primary amoebic meningoencephalitis.[15]
Spectrum of susceptibility
[edit]The following table shows the amphotericin B susceptibility for a selection of medically important fungi.
| Species | Amphotericin B
MIC breakpoint (mg/L) |
|---|---|
| Aspergillus fumigatus | 1[16] |
| Aspergillus terreus | Resistant[16][17] |
| Candida albicans | 1[16] |
| Candida glabrata | 1[16] |
| Candida krusei | 1[16] |
| Candida lusitaniae | Intrinsically resistant[17] |
| Cryptococcus neoformans | 2[18] |
| Fusarium oxysporum | 2[18] |
Available formulations
[edit]Intravenous
[edit]Amphotericin B alone is insoluble in normal saline at a pH of 7. Therefore, several formulations have been devised to improve its intravenous bioavailability.[19] Lipid-based formulations of amphotericin B are no more effective than conventional formulations, although there is some evidence that lipid-based formulations may be better tolerated by patients and may have fewer adverse effects.[20]
Deoxycholate
[edit]The original formulation uses sodium deoxycholate to improve solubility.[17] Amphotericin B deoxycholate (ABD) is administered intravenously.[21] As the original formulation of amphotericin, it is often referred to as "conventional" amphotericin.[22]
Liposomal
[edit]In order to improve the tolerability of amphotericin and reduce toxicity, several lipid formulations have been developed.[17] Liposomal formulations have been found to have less renal toxicity than deoxycholate,[23][24] and fewer infusion-related reactions.[17] They are more expensive than amphotericin B deoxycholate.[25]
AmBisome (liposomal amphotericin B; LAMB) is a liposomal formulation of amphotericin B for injection and consists of a mixture of phosphatidylcholine, cholesterol and distearoyl phosphatidylglycerol that in aqueous media spontaneously arrange into unilamellar vesicles that contain amphotericin B.[17][26] It was developed by NeXstar Pharmaceuticals (acquired by Gilead Sciences in 1999). It was approved by the FDA in 1997.[27] It is marketed by Gilead in Europe and licensed to Astellas Pharma (formerly Fujisawa Pharmaceuticals) for marketing in the US, and Sumitomo Pharmaceuticals in Japan.[citation needed]
Lipid complex formulations
[edit]A number of lipid complex preparations are also available. Abelcet was approved by the FDA in 1995.[28] It consists of amphotericin B and two lipids in a 1:1 ratio that form large ribbon-like structures.[17] Amphotec is a complex of amphotericin and sodium cholesteryl sulfate in a 1:1 ratio. Two molecules of each form a tetramer that aggregate into spiral arms on a disk-like complex.[26] It was approved by the FDA in 1996.[28]
By mouth
[edit]An oral preparation exists but is not widely available.[29] The amphipathic nature of amphotericin along with its low solubility and permeability has posed major hurdles for oral administration given its low bioavailability. In the past it had been used for fungal infections of the surface of the GI tract such as thrush, but has been replaced by other antifungals such as nystatin and fluconazole.[30]
However, recently novel nanoparticulate drug delivery systems such as AmbiOnp,[31] nanosuspensions, lipid-based drug delivery systems including cochleates, self-emulsifying drug delivery systems,[32] solid lipid nanoparticles[31] and polymeric nanoparticles[33]—such as amphotericin B in pegylated polylactide coglycolide copolymer nanoparticles[34]—have demonstrated potential for oral formulation of amphotericin B.[35] The oral lipid nanocrystal amphotericin by Matinas Biopharma is furthest along having completed a successful phase 2 clinical trial in cryptococcal meningitis.[36]
Side effects
[edit]Amphotericin B is well known for its severe and potentially lethal side effects, earning it the nickname "amphoterrible".[37][38] Very often, it causes a serious reaction soon after infusion (within 1 to 3 hours), consisting of high fever, shaking chills, hypotension, anorexia, nausea, vomiting, headache, dyspnea and tachypnea, drowsiness, and generalized weakness. The violent chills and fevers have caused the drug to be nicknamed "shake and bake".[39][40] The precise etiology of the reaction is unclear, although it may involve increased prostaglandin synthesis and the release of cytokines from macrophages.[41][42] Deoxycholate formulations (ABD) may also stimulate the release of histamine from mast cells and basophils.[43] Reactions sometimes subside with later applications of the drug. This nearly universal febrile response necessitates a critical (and diagnostically difficult) professional determination as to whether the onset of high fever is a novel symptom of a fast-progressing disease, or merely the effect of the drug. To decrease the likelihood and severity of the symptoms, initial doses should be low, and increased slowly. Paracetamol, pethidine, diphenhydramine, and hydrocortisone have all been used to treat or prevent the syndrome, but the prophylactic use of these drugs is often limited by the patient's condition.[44]
Intravenously administered amphotericin B in therapeutic doses has also been associated with multiple organ damage. Kidney damage is a frequently reported side effect, and can be severe and/or irreversible. Less kidney toxicity has been reported with liposomal formulations (such as AmBisome) and it has become preferred in patients with preexisting renal injury.[45][46] The integrity of the liposome is disrupted when it binds to the fungal cell wall, but is not affected by the mammalian cell membrane,[47] so the association with liposomes decreases the exposure of the kidneys to amphotericin B, which explains its less nephrotoxic effects.[48]
In addition, electrolyte imbalances such as hypokalemia and hypomagnesemia are also common.[49] In the liver, increased liver enzymes and hepatotoxicity (up to and including fulminant liver failure) are common. In the circulatory system, several forms of anemia and other blood dyscrasias (leukopenia, thrombopenia), serious cardiac arrhythmias (including ventricular fibrillation), and even frank cardiac failure have been reported. Skin reactions, including serious forms, are also possible.[citation needed]
Interactions
[edit]Drug-drug interactions may occur when amphotericin B is coadministered with the following agents:[50]
- Flucytosine: Toxicity of flucytosine is increased and allows a lower dose of amphotericin B. Amphotericin B may also facilitate entry of flucystosine into the fungal cell by interfering with the permeability of the fungal cell membrane.
- Diuretics or cisplatin: Increased renal toxicity and increased risk of hypokalemia
- Corticosteroids: Increased risk of hypokalemia
- Imidazole Antifungals: Amphotericin B may antagonize the activity of ketoconazole and miconazole. The clinical significance of this interaction is unknown.
- Neuromuscular-blocking agents: Amphotericin B-induced hypokalemia may potentiate the effects of certain paralytic agents.
- Foscarnet, ganciclovir, tenofovir, adefovir: Risk of hematological and renal side effects of amphotericin B are increased
- Zidovudine: Increased risk of renal and hematological toxicity .
- Other nephrotoxic drugs (such as aminoglycosides): Increased risk of serious renal damage
- Cytostatic drugs: Increased risk of kidney damage, hypotension, and bronchospasms
- Transfusion of leukocytes: Risk of pulmonal (lung) damage occurs, space the intervals between the application of amphotericin B and the transfusion, and monitor pulmonary function
Mechanism of action
[edit]Amphotericin B binds with ergosterol, a component of fungal cell membranes, forming pores that cause rapid leakage of monovalent ions (K+, Na+, H+ and Cl−) and subsequent fungal cell death. This is amphotericin B's primary effect as an antifungal agent.[51][52] It has been found that the amphotericin B/ergosterol bimolecular complex that maintains these pores is stabilized by Van der Waals interactions.[53] Researchers have found evidence that amphotericin B also causes oxidative stress within the fungal cell,[54] but it remains unclear to what extent this oxidative damage contributes to the drug's effectiveness.[51] The addition of free radical scavengers or antioxidants can lead to amphotericin resistance in some species, such as Scedosporium prolificans, without affecting the cell wall.[citation needed]
Two amphotericins, amphotericin A and amphotericin B, are known, but only B is used clinically, because it is significantly more active in vivo. Amphotericin A is almost identical to amphotericin B (having a C=C double bond between the 27th and 28th carbons), but has little antifungal activity.[19]
Mechanism of toxicity
[edit]Mammalian and fungal membranes both contain sterols, a primary membrane target for amphotericin B. Because mammalian and fungal membranes are similar in structure and composition, this is one mechanism by which amphotericin B causes cellular toxicity. Amphotericin B molecules can form pores in the host membrane as well as the fungal membrane. This impairment in membrane barrier function can have lethal effects.[54][55][56] Ergosterol, the fungal sterol, is more sensitive to amphotericin B than cholesterol, the common mammalian sterol. Reactivity with the membrane is also sterol concentration dependent.[57] Bacteria are not affected as their cell membranes do not usually contain sterols.[citation needed]
Amphotericin B administration is limited by infusion-related toxicity. This is thought to result from innate immune production of proinflammatory cytokines.[55][58]
Biosynthesis
[edit]The natural route to synthesis includes polyketide synthase components.[59] The carbon chains of amphotericin B are assembled from sixteen 'C2' acetate and three 'C3'propionate units by polyketide syntheses (PKSs).[60] Polyketide biosynthesis begins with the decarboxylative condensation of a dicarboxylic acid extender unit with a starter acyl unit to form a β-ketoacyl intermediate. The growing chain is constructed by a series of Claisen reactions. Within each module, the extender units are loaded onto the current ACP domain by acetyl transferase (AT). The ACP-bound elongation group reacts in a Claisen condensation with the KS-bound polyketide chain. Ketoreductase (KR), dehydratase (DH) and enoyl reductase (ER) enzymes may also be present to form alcohol, double bonds or single bonds.[61] After cyclisation, the macrolactone core undergoes further modification by hydroxylation, methylation and glycosylation. The order of these three post-cyclization processes is unknown.[61]
History
[edit]It was originally extracted from Streptomyces nodosus, a filamentous bacterium, in 1955, at the Squibb Institute for Medical Research from cultures of an undescribed streptomycete isolated from the soil collected in the Orinoco River region of Venezuela.[19][62] Two antifungal substances were isolated from the soil culture, amphotericin A and amphotericin B, but B had better antifungal activity. For decades it remained the only effective therapy for invasive fungal disease until the development of the azole antifungals in the early 1980s.[21]
Its complete stereo structure was determined in 1970 by an X-ray structure of the N-iodoacetyl derivative.[60] The first synthesis of the compound's naturally occurring enantiomeric form was achieved in 1987 by K. C. Nicolaou.[63]
Amphotericin B was used to treat a patient with disseminated coccidioidomycosis who was admitted to the U.S. Public Health Service Hospital, Seattle, Washington on January 16, 1957. "The course was rapidly downhill with a grim prognosis as manifested by positive blood cultures, rising complement fixation titers, and failure of the skin to react to intradermal coccidioidin. Amphotericin B was started eight weeks following the onset of his illness. Following this there was remarkable improvement both objectively and subjectively. A fourteen-month follow-up following discontinuance of the drug revealed stabilization of all laboratory studies except for a re-elevation of the complement fixation titer from 1 to 16 to 1 to 32. The patient was completely asymptomatic except for the production of sputum containing a few spherules. The clinical effect of this drug in this patient has been most encouraging and is in agreement with results obtained by others. The lasting effect of the drug seems suggested by the patient's complete well-being after fourteen months of cessation of treatment. It is reasonable to assume that this drug will play a major part in the specific treatment of this disease." [64]
Formulations
[edit]It is a subgroup of the macrolide antibiotics, and exhibits similar structural elements.[65] Currently, the drug is available in many forms. Either "conventionally" complexed with sodium deoxycholate (ABD), as a cholesteryl sulfate complex (ABCD), as a lipid complex (ABLC), and as a liposomal formulation (LAMB). The latter formulations have been developed to improve tolerability and decrease toxicity, but may show considerably different pharmacokinetic characteristics compared to conventional amphotericin B.[17]
Names
[edit]Amphotericin's name originates from the chemical's amphoteric properties.[66]
It is commercially known as Fungilin, Fungizone, Abelcet, AmBisome, Fungisome, Amphocil, Amphotec, and Halizon.[67]
References
[edit]- ^ "Updates to the Prescribing Medicines in Pregnancy database". Therapeutic Goods Administration (TGA). 12 May 2022. Retrieved 13 May 2022.
- ^ "Ambisome- amphotericin b injection, powder, lyophilized, for solution". DailyMed. Retrieved 11 August 2021.
- ^ a b c World Health Organization (March 2010). Control of the leishmaniasis: report of a meeting of the WHO Expert Committee on the Control of Leishmaniases. Geneva: World Health Organization. pp. 55, 88, 186. hdl:10665/44412. ISBN 9789241209496.
- ^ a b c d e f g h "Amphotericin B". The American Society of Health-System Pharmacists. Archived from the original on 2015-01-01. Retrieved January 1, 2015.
- ^ World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 145. hdl:10665/44053. ISBN 9789241547659.
- ^ Velázquez L (1976). Farmacología y su proyección a la clínica 13a ed (in Spanish). Oteo. p. 966. ISBN 8485152050.
- ^ Walker SR (2012). Trends and Changes in Drug Research and Development. Springer Science & Business Media. p. 109. ISBN 9789400926592. Archived from the original on 2017-09-10.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 477. ISBN 9783527607495.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ "Competitive Generic Therapy Approvals". U.S. Food and Drug Administration (FDA). 29 June 2023. Archived from the original on 29 June 2023. Retrieved 29 June 2023.
- ^ Bennett JE, Dolin R, Blaser MJ (28 August 2014). Drugs Active against Fungi, Pneumocystis, and Microsporidia. Elsevier Health Sciences. pp. 479–494.e4. ISBN 978-1-4557-4801-3.
- ^ Moen MD, Lyseng-Williamson KA, Scott LJ (2012-09-17). "Liposomal amphotericin B: a review of its use as empirical therapy in febrile neutropenia and in the treatment of invasive fungal infections". Drugs. 69 (3): 361–392. doi:10.2165/00003495-200969030-00010. PMID 19275278. S2CID 34340503.
- ^ Rura N (2013-10-29). "Understanding the evolution of drug resistance points to novel strategy for developing better antimicrobials". Archived from the original on 2016-11-15. Retrieved 2016-11-14 – via Whitehead Institute.
- ^ den Boer M, Davidson RN (April 2006). "Treatment options for visceral leishmaniasis". Expert Review of Anti-Infective Therapy. 4 (2): 187–197. doi:10.1586/14787210.4.2.187. PMID 16597201. S2CID 42784356.
- ^ Grace E, Asbill S, Virga K (November 2015). "Naegleria fowleri: pathogenesis, diagnosis, and treatment options". Antimicrobial Agents and Chemotherapy. 59 (11): 6677–6681. doi:10.1128/AAC.01293-15. PMC 4604384. PMID 26259797.
- ^ a b c d e European Committee on Antimicrobial Susceptibility Testing (2015-11-16). "Antifungal Agents, Breakpoint tables for interpretation of MICs" (PDF). Retrieved 2015-11-17.
- ^ a b c d e f g h Hamill RJ (June 2013). "Amphotericin B formulations: a comparative review of efficacy and toxicity". Drugs. 73 (9): 919–934. doi:10.1007/s40265-013-0069-4. PMID 23729001. S2CID 2785865.
- ^ a b "Index | The Antimicrobial Index Knowledgebase - TOKU-E". antibiotics.toku-e.com. Archived from the original on 2015-11-09. Retrieved 2015-11-17.
- ^ a b c Dutcher JD (October 1968). "The discovery and development of amphotericin B". Diseases of the Chest. 54 (Supplement_1): 296–298. doi:10.1378/chest.54.Supplement_1.296. PMID 4877964.
- ^ Steimbach, Laiza M., Fernanda S. Tonin, Suzane Virtuoso, Helena HL Borba, Andréia CC Sanches, Astrid Wiens, Fernando Fernandez-Llimós, and Roberto Pontarolo. "Efficacy and safety of amphotericin B lipid-based formulations—A systematic review and meta-analysis." Mycoses 60, no. 3 (2017): 146-154.
- ^ a b Maertens JA (March 2004). "History of the development of azole derivatives". Clinical Microbiology and Infection. 10 (Suppl 1): 1–10. doi:10.1111/j.1470-9465.2004.00841.x. PMID 14748798.
- ^ Clemons KV, Stevens DA (April 1998). "Comparison of fungizone, Amphotec, AmBisome, and Abelcet for treatment of systemic murine cryptococcosis". Antimicrobial Agents and Chemotherapy. 42 (4): 899–902. doi:10.1128/AAC.42.4.899. PMC 105563. PMID 9559804.
- ^ Botero Aguirre JP, Restrepo Hamid AM (November 2015). "Amphotericin B deoxycholate versus liposomal amphotericin B: effects on kidney function". The Cochrane Database of Systematic Reviews. 2015 (11): CD010481. doi:10.1002/14651858.cd010481.pub2. PMC 10542271. PMID 26595825.
- ^ Mistro S, Maciel I, de Menezes RG, Maia ZP, Schooley RT, Badaró R (June 2012). "Does lipid emulsion reduce amphotericin B nephrotoxicity? A systematic review and meta-analysis". Clinical Infectious Diseases. 54 (12): 1774–1777. doi:10.1093/cid/cis290. PMID 22491505.
- ^ Bennett J (November 2000). "Editorial response: choosing amphotericin B formulations-between a rock and a hard place". Clinical Infectious Diseases. 31 (5): 1164–1165. doi:10.1086/317443. PMID 11073746.
- ^ a b Slain D (March 1999). "Lipid-based amphotericin B for the treatment of fungal infections". Pharmacotherapy. 19 (3): 306–323. doi:10.1592/phco.19.4.306.30934. PMID 10221369. S2CID 43479677.
- ^ "Drug Approval Package". www.accessdata.fda.gov. Archived from the original on 2015-11-17. Retrieved 2015-11-03.
- ^ a b "Drugs@FDA: FDA Approved Drug Products". www.accessdata.fda.gov. Archived from the original on 2014-08-13. Retrieved 2015-11-03.
- ^ Wasan KM, Wasan EK, Gershkovich P, Zhu X, Tidwell RR, Werbovetz KA, et al. (August 2009). "Highly effective oral amphotericin B formulation against murine visceral leishmaniasis". The Journal of Infectious Diseases. 200 (3): 357–360. doi:10.1086/600105. PMID 19545212.
- ^ Pappas PG, Kauffman CA, Andes D, Benjamin DK, Calandra TF, Edwards JE, et al. (March 2009). "Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America". Clinical Infectious Diseases. 48 (5): 503–535. doi:10.1086/596757. PMC 7294538. PMID 19191635.
- ^ a b Patel PA, Patravale VB (October 2011). "AmbiOnp: solid lipid nanoparticles of amphotericin B for oral administration". Journal of Biomedical Nanotechnology. 7 (5): 632–639. doi:10.1166/jbn.2011.1332. PMID 22195480.
- ^ Wasan EK, Bartlett K, Gershkovich P, Sivak O, Banno B, Wong Z, et al. (May 2009). "Development and characterization of oral lipid-based amphotericin B formulations with enhanced drug solubility, stability and antifungal activity in rats infected with Aspergillus fumigatus or Candida albicans". International Journal of Pharmaceutics. 372 (1–2): 76–84. doi:10.1016/j.ijpharm.2009.01.003. PMID 19236839.
- ^ Italia JL, Yahya MM, Singh D, Ravi Kumar MN (June 2009). "Biodegradable nanoparticles improve oral bioavailability of amphotericin B and show reduced nephrotoxicity compared to intravenous Fungizone". Pharmaceutical Research. 26 (6): 1324–1331. doi:10.1007/s11095-009-9841-2. PMID 19214716. S2CID 8612917.
- ^ Al-Quadeib BT, Radwan MA, Siller L, Horrocks B, Wright MC (July 2015). "Stealth Amphotericin B nanoparticles for oral drug delivery: In vitro optimization". Saudi Pharmaceutical Journal. 23 (3): 290–302. doi:10.1016/j.jsps.2014.11.004. PMC 4475820. PMID 26106277.
- ^ Patel PA, Fernandes CB, Pol AS, Patravale VB (2013). "Oral amphotericin B: challenges and avenues". Int. J. Pharm. Biosci. Technol. 1 (1): 1–9.
- ^ Boulware DR, Atukunda M, Kagimu E, Musubire AK, Akampurira A, Tugume L, et al. (August 2023). "Oral Lipid Nanocrystal Amphotericin B for Cryptococcal Meningitis: A Randomized Clinical Trial". Clinical Infectious Diseases. 77 (12): 1659–1667. doi:10.1093/cid/ciad440. PMC 10724459. PMID 37606364.
- ^ Carr JR, Hawkins WA, Newsome AS, Smith SE, Amber BC, Bland CM, et al. (October 2022). "Fluid Stewardship of Maintenance Intravenous Fluids". Journal of Pharmacy Practice. 35 (5): 769–782. doi:10.1177/08971900211008261. PMC 8497650. PMID 33827313.
- ^ Mourad A, Perfect JR (January 2018). "Tolerability profile of the current antifungal armoury". The Journal of Antimicrobial Chemotherapy. 73 (suppl_1): i26 – i32. doi:10.1093/jac/dkx446. PMC 6636388. PMID 29304209.
- ^ "Shake and Bake". TheFreeDictionary.com. Retrieved 2016-12-09.
- ^ Hartsel SC. "Studies on Amphotericin B" (PDF). Chem 491, Chemistry Department. University of Wisconsin-Eau Claire. Archived (PDF) from the original on 20 December 2016. Retrieved 8 December 2016.
- ^ Gigliotti F, Shenep JL, Lott L, Thornton D (November 1987). "Induction of prostaglandin synthesis as the mechanism responsible for the chills and fever produced by infusing amphotericin B". The Journal of Infectious Diseases. 156 (5): 784–789. doi:10.1093/infdis/156.5.784. PMID 3309074.
- ^ Sau K, Mambula SS, Latz E, Henneke P, Golenbock DT, Levitz SM (September 2003). "The antifungal drug amphotericin B promotes inflammatory cytokine release by a Toll-like receptor- and CD14-dependent mechanism". The Journal of Biological Chemistry. 278 (39): 37561–37568. doi:10.1074/jbc.M306137200. PMID 12860979.
- ^ Baronti R, Masini E, Bacciottini L, Mannaioni PF (May 2002). "Differential effects of amphotericin B and liposomal amphotericin B on inflammatory cells in vitro". Inflammation Research. 51 (5): 259–264. doi:10.1007/pl00000302. PMID 12056514. S2CID 2124507.
- ^ Goodwin SD, Cleary JD, Walawander CA, Taylor JW, Grasela TH (April 1995). "Pretreatment regimens for adverse events related to infusion of amphotericin B". Clinical Infectious Diseases. 20 (4): 755–761. doi:10.1093/clinids/20.4.755. PMID 7795069.
- ^ Walsh TJ, Finberg RW, Arndt C, Hiemenz J, Schwartz C, Bodensteiner D, et al. (March 1999). "Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. National Institute of Allergy and Infectious Diseases Mycoses Study Group". The New England Journal of Medicine. 340 (10): 764–771. doi:10.1056/NEJM199903113401004. PMID 10072411.
- ^ Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, et al. (February 2010). "Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america". Clinical Infectious Diseases. 50 (3): 291–322. doi:10.1086/649858. PMC 5826644. PMID 20047480.
- ^ Jill Adler-Moore,* and Richard T. liposomal formulation, structure, mechanism of action and pre-clinical experience. Journal of Antimicrobial Chemotherapy (2002) 49, 21–30
- ^ J. Czub, M. Baginski. Amphotericin B and Its New Derivatives Mode of action. Department of pharmaceutical Technology and Biochemistry. Faculty of Chemistry, Gdnsk University of Technology. 2009, 10-459-469.
- ^ Zietse R, Zoutendijk R, Hoorn EJ (April 2009). "Fluid, electrolyte and acid-base disorders associated with antibiotic therapy". Nature Reviews. Nephrology. 5 (4): 193–202. doi:10.1038/nrneph.2009.17. PMID 19322184. S2CID 24486546.
- ^ "Abelcet Package Insert" (PDF). Leadiant Biosciences. Sigma-Tau Pharmaceuticals. Retrieved 14 July 2022.
- ^ a b Mesa-Arango AC, Scorzoni L, Zaragoza O (2012-01-01). "It only takes one to do many jobs: Amphotericin B as antifungal and immunomodulatory drug". Frontiers in Microbiology. 3: 286. doi:10.3389/fmicb.2012.00286. PMC 3441194. PMID 23024638.
- ^ O'Keeffe J, Doyle S, Kavanagh K (December 2003). "Exposure of the yeast Candida albicans to the anti-neoplastic agent adriamycin increases the tolerance to amphotericin B" (PDF). The Journal of Pharmacy and Pharmacology. 55 (12): 1629–1633. doi:10.1211/0022357022359. PMID 14738588. S2CID 38893122.
- ^ Baran M, Borowski E, Mazerski J (May 2009). "Molecular modeling of amphotericin B-ergosterol primary complex in water II" (PDF). Biophysical Chemistry. 141 (2–3): 162–168. doi:10.1016/j.bpc.2009.01.010. PMID 19233539.
- ^ a b Baginski M, Czub J (June 2009). "Amphotericin B and its new derivatives - mode of action". Current Drug Metabolism. 10 (5): 459–469. doi:10.2174/138920009788898019. PMID 19689243.
- ^ a b Laniado-Laborín R, Cabrales-Vargas MN (December 2009). "Amphotericin B: side effects and toxicity". Revista Iberoamericana de Micologia. 26 (4): 223–7. doi:10.1016/j.riam.2009.06.003. PMID 19836985. S2CID 592301.
- ^ "Amphocin, amphotericin B for injection, USP" (PDF). Pfizer. Archived from the original (PDF) on 2011-04-19. Retrieved 2010-02-18.
- ^ Vertut-Croquin A, Bolard J, Chabbert M, Gary-Bobo C (June 1983). "Differences in the interaction of the polyene antibiotic amphotericin B with cholesterol- or ergosterol-containing phospholipid vesicles. A circular dichroism and permeability study". Biochemistry. 22 (12): 2939–2944. doi:10.1021/bi00281a024. PMID 6871175.
- ^ Drew RH, Kauffman CA, Thorner AR. "Pharmacology of amphotericin B." UpToDate. MA Waltham.
- ^ Khan N, Rawlings B, Caffrey P (June 2011). "A labile point in mutant amphotericin polyketide synthases". Biotechnology Letters. 33 (6): 1121–1126. doi:10.1007/s10529-011-0538-3. PMID 21267757. S2CID 10209476.
- ^ a b McNamara C, Crawforth J, Hickman B, Norwood T, Rawlings B (January 1998). "Biosynthesis of amphotericin B" (PDF). Journal of the Chemical Society, Perkin Transactions 1 (thesis) (1): 83–88. doi:10.1039/A704545J. hdl:2381/33805. Archived from the original (PDF) on 2017-09-21. Retrieved 2018-05-16.
- ^ a b Caffrey P, Lynch S, Flood E, Finnan S, Oliynyk M (July 2001). "Amphotericin biosynthesis in Streptomyces nodosus: deductions from analysis of polyketide synthase and late genes". Chemistry & Biology. 8 (7): 713–723. doi:10.1016/S1074-5521(01)00046-1. PMID 11451671.
- ^ Procópio RE, Silva IR, Martins MK, Azevedo JL, Araújo JM (2012). "Antibiotics produced by Streptomyces". The Brazilian Journal of Infectious Diseases. 16 (5): 466–471. doi:10.1016/j.bjid.2012.08.014. PMID 22975171.
- ^ Nicolaou KC, Daines RA, Chakraborty TK, Ogawa Y (1987-04-01). "Total synthesis of amphotericin B". Journal of the American Chemical Society. 109 (9): 2821–2822. doi:10.1021/ja00243a043. ISSN 0002-7863.
- ^ Salvatore A. La Barbera, M.D. New York State Journal of Medicine, Vol. 59, No. 19, Oct, 1, 1959.
- ^ "Chemistry and Biology of the Polyene Macrolide Antibiotics". Bacteriological Reviews. 32.
- ^ Christine D. Waugh, in xPharm: The Comprehensive Pharmacology Reference, 2007.
- ^ "Halizon". Edu.drugs. Archived from the original on 2016-11-15. Retrieved 2016-11-14.
