Aminopolycarboxylic acid

An aminopolycarboxylic acid (sometimes abbreviated APCA) is a chemical compound containing one or more nitrogen atoms connected through carbon atoms to two or more carboxyl groups. Aminopolycarboxylates that have lost acidic protons form strong complexes with metal ions. This property makes aminopolycarboxylic acids useful complexone in a wide variety of chemical, medical, and environmental applications.[1]
Structure
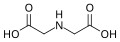
[edit]The parent of this family of ligands is the amino acid glycine, H2NCH2COOH, in which the amino group, NH2, is separated from the carboxyl group, COOH by a single methylene group, CH2. When the carboxyl group is deprotonated the glycinate ion can function as a bidentate ligand, binding the metal centre through the nitrogen and one of two carboxylate oxygen atoms, to form chelate complexes of metal ions.[2]
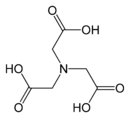
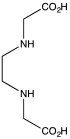
Replacement of a hydrogen atom on the nitrogen of glycine by another acetate residue, –CH2COOH gives iminodiacetic acid, IDA, which is a tridentate ligand. Further substitution gives nitrilotriacetic acid, NTA, which is a tetradentate ligand.[3] These compounds can be described as aminopolycarboxylates. Related ligands can be derived from other amino acids other than glycine, notably aspartic acid.

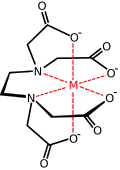
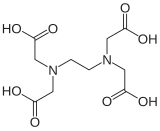
Higher density is achieved by linking two or more glycinate or IDA units together. EDTA contains two IDA units with the nitrogen atoms linked by two methylene groups and is hexadentate. DTPA has two CH2CH2 bridges linking three nitrogen atoms and is octadentate. TTHA[1] has ten potential donor atoms.
Applications
[edit]The chelating properties of aminopolycarboxylates can be engineered by varying the groups linking the nitrogen atoms so as to increase selectivity for a particular metal ion. The number of carbon atoms between the nitrogen and carboxyl group can also be varied and substituents can be placed on these carbon atoms. Altogether this allows for a vast range of possibilities. Fura-2 combines two functionalities: it has high selectivity for calcium over magnesium and it has a substituent which makes the complex fluorescent when it binds calcium. This reagent provides a means of determining the calcium content in intra-cellular fluid. Details concerning applications of the following examples can be found in the individual articles and/or reference. The aminopolycarboxylate nicotianamine is widespread in plants, where it is used to transport iron.
 |
 |
 | |
| glycinate | IDA[1] | NTA[3] | EDDA[3] |
|
|

|

|
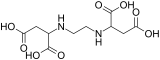
| EDTA | EDDS | DTPA[1] | EGTA |

|

|
| |
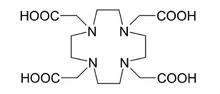
| BAPTA | NOTA[1] | DOTA[1] | |

|

| ||
| Nicotianamine[4] | EDDHA |
References
[edit]- ^ a b c d e f Anderegg, G.; Arnaud-Neu, F.; Delgado, R.; Felcman, J.; Popov, K. (2005). "Critical evaluation of stability constants of metal complexes of complexones for biomedical and environmental applications* (IUPAC Technical Report)". Pure Appl. Chem. 77 (8): 1445–1495. doi:10.1351/pac200577081445. hdl:20.500.11850/423005. pdf
- ^ Schwarzenbach, G. (1952). "Der Chelateffekt". Helv. Chim. Acta. 35 (7): 2344–2359. doi:10.1002/hlca.19520350721.
- ^ a b c Anderegg, G (1982). "Critical survey of stability constants of NTA complexes". Pure Appl. Chem. 54 (12): 2693–2758. doi:10.1351/pac198254122693. pdf
- ^ Curie, C.; Cassin, G.; Couch, D.; Divol, F.; Higuchi, K.; Le Jean, M.; Misson, J.; Schikora, A.; Czernic, P.; Mari, S. (2009). "Metal movement within the plant: contribution of nicotianamine and yellow stripe 1-like transporters". Annals of Botany. 103 (1): 1–11. doi:10.1093/aob/mcn207. PMC 2707284. PMID 18977764.