Glycerine acetate
Appearance
(Redirected from Acetine)
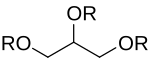 R = H or CH3C(=O)
| |
| Names | |
|---|---|
| Other names
glycerol acetate
glyceryl acetate 1,2,3-propanetriol acetate | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.014.216 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
| |
| |
| Properties | |
| Variable | |
| Molar mass | Variable |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Glycerine acetate is a mixture of esters produced from the esterification of glycerol (1) with acetic acid. This reaction produces five congeners:
- the two monoacetylglycerols / MAG / monoacetin (2 and 3)
- the two diacetylglycerols / DAG / diacetin / glyceryl diacetate (4 and 5)
- the one triacetalglycerol / TAG / triacetin (6)
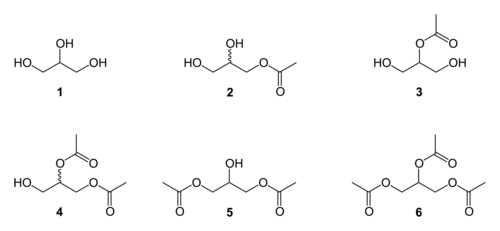
In addition, two of the congeners, 2 and 4, are chiral and can exist in either of two enantiomeric forms.
Uses
[edit]DAG and TAG can be used as fuel additives for improving the cold and viscosity properties of biodiesel or the antiknocking properties of gasoline.[1]
Notes
[edit]- ^ J. A. Melero; R. vanGrieken; G. Morales; M. Paniagua (2007). "Acidic Mesoporous Silica for the Acetylation of Glycerol: Synthesis of Bioadditives to Petrol Fuel". Energy & Fuels. 21 (3): 1782–1791. doi:10.1021/ef060647q.
