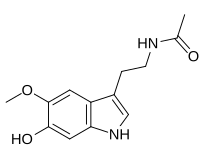
6-Hydroxymelatonin
| |
| Names | |
|---|---|
| Preferred IUPAC name
N-[2-(6-Hydroxy-5-methoxy-1H-indol-3-yl)ethyl]acetamide | |
| Other names
6-Oxymelatonin
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.164.426 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C13H16N2O3 | |
| Molar mass | 248.282 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
6-Hydroxymelatonin (6-OHM) is a naturally occurring, endogenous, major active metabolite of melatonin.[1] 6-Hydroxymelatonin is produced as a result of the enzymatic conversion of melatonin through hydroxylation.[2] Similar to melatonin, 6-OHM is a full agonist of the MT1 and MT2 receptors.[3][4] It is also an antioxidant and neuroprotective, and is even more potent in this regard relative to melatonin.[5][6]
Role in metabolism
[edit]The determination of 6-OHM in human urine has been used to track the metabolism and excretion of melatonin using LC-MS/MS, providing quantifiable insights into circadian rhythm regulation and its oxidative role as a biomarker.[7] 6-OHM is one of four of the primary metabolic products of melatonin in the liver and is also a byproduct of its breakdown due to exposure to light. It is known to be very effective in protecting cells from oxidative damage caused by ultraviolet (UV) radiation.[8] Based on comparisons with other melatonin-related compounds, it is suggested that the protective effects of 6-OHM in mitigating oxidative stress are primarily attributed to their ability to scavenge free radicals.[9]
See also
[edit]- N-Acetylserotonin (normelatonin)
- 5-Methoxytryptamine
- Melatonin
References
[edit]- ^ Hardeland R (2010). "Melatonin metabolism in the central nervous system". Curr Neuropharmacol. 8 (3): 168–81. doi:10.2174/157015910792246164. PMC 3001211. PMID 21358968.
- ^ Magliocco, G.; Le Bloc'h, F.; Thomas, A.; Desmeules, J.; Daali, Y. (2021-09-01). "Simultaneous determination of melatonin and 6-hydroxymelatonin in human overnight urine by LC-MS/MS". Journal of Chromatography. 1181: 122938. doi:10.1016/j.jchromb.2021.122938.
- ^ Dubocovich ML (1988). "Pharmacology and function of melatonin receptors". FASEB J. 2 (12): 2765–73. doi:10.1096/fasebj.2.12.2842214. PMID 2842214. S2CID 45788574.
- ^ Browning, Christopher; Beresford, Isabel; Fraser, Neil; Giles, Heather (2000). "Pharmacological characterization of human recombinant melatonin mt1and MT2receptors". British Journal of Pharmacology. 129 (5): 877–886. doi:10.1038/sj.bjp.0703130. ISSN 0007-1188. PMC 1571913. PMID 10696085.
- ^ Maharaj DS, Glass BD, Daya S (2007). "Melatonin: new places in therapy". Biosci. Rep. 27 (6): 299–320. doi:10.1007/s10540-007-9052-1. PMID 17828452. S2CID 32437175.
- ^ Álvarez-Diduk R, Galano A, Tan DX, Reiter RJ (2015). "N-Acetylserotonin and 6-Hydroxymelatonin against Oxidative Stress: Implications for the Overall Protection Exerted by Melatonin". J Phys Chem B. 119 (27): 8535–43. doi:10.1021/acs.jpcb.5b04920. PMID 26079042.
- ^ Magliocco, G.; Le Bloc'h, F.; Thomas, A.; Desmeules, J.; Daali, Y. (2021-09-01). "Simultaneous determination of melatonin and 6-hydroxymelatonin in human overnight urine by LC-MS/MS". Journal of Chromatography B. 1181: 122938. doi:10.1016/j.jchromb.2021.122938.
- ^ Maharaj, Deepa S.; Anoopkumar‐Dukie, Shailendra; Glass, Beverley D.; Antunes, Edith M.; Lack, Barbara; Walker, Roderick B.; Daya, Santy (2002-04-30). "The identification of the UV degradants of melatonin and their ability to scavenge free radicals". Journal of Pineal Research. 32 (4): 257–261. doi:10.1034/j.1600-079x.2002.01866.x. ISSN 0742-3098.
- ^ Álvarez-Diduk, Ruslán; Galano, Annia; Tan, Dun Xian; Reiter, Russel J. (2015-07-09). "N -Acetylserotonin and 6-Hydroxymelatonin against Oxidative Stress: Implications for the Overall Protection Exerted by Melatonin". The Journal of Physical Chemistry B. 119 (27): 8535–8543. doi:10.1021/acs.jpcb.5b04920. ISSN 1520-6106.
