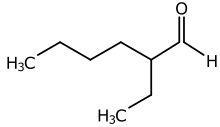
2-Ethylhexanal
Appearance
(Redirected from 2-ethylhexanal)
| |
| Names | |
|---|---|
| IUPAC name
2-Ethylhexanal
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.179 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 1191 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H16O | |
| Molar mass | 128.215 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.820 g/cm3 |
| Melting point | <-60 |
| Boiling point | 163 °C (325 °F; 436 K) |
Refractive index (nD)
|
1.416 |
| Hazards | |
| GHS labelling:[1] | |
  
| |
| Warning | |
| H226, H315, H317, H319, H361 | |
| P203, P210, P233, P240, P241, P242, P243, P261, P264, P264+P265, P272, P280, P302+P352, P303+P361+P353, P305+P351+P338, P318, P321, P332+P317, P333+P317, P337+P317, P362+P364, P370+P378, P403+P235, P405, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2-Ethylhexanal is the organic compound with the formula CH3CH2CH2CH2CH(C2H5)CHO. A colorless liquid, it is produced on a large scale industrially as a precursor to 2-ethylhexanoic acid and 2-ethylhexanol, both used as precursors to plasticizers. It was studied in the detergent industry since the 1930s.[2]
2-Ethylhexanal is synthesized by aldol condensation of two equivalents of butyraldehyde followed by hydrogenation of the intermediate 2-ethylhexenal.[3] The compound is chiral, but is mainly used as a racemic mixture.
References
[edit]- ^ "2-Ethylhexanal". pubchem.ncbi.nlm.nih.gov.
- ^ Gangloff, W. C. (1938). "Changing trends in detergents". Oil and Soap. 15 (1): 14–17. doi:10.1007/BF02549560. ISSN 2331-3420.
- ^ Kohlpaintner, Christian; Schulte, Markus; Falbe, Jürgen; Lappe, Peter; Weber, Jürgen (2008). "Aldehydes, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a01_321.pub2. ISBN 978-3-527-30673-2.

