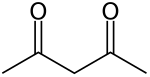
Acetylacetone
| |||
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
(3Z)-4-Hydroxy-3-penten-2-one (enol form)
Pentane-2,4-dione (keto form) | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 741937 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.214 | ||
| EC Number |
| ||
| 2537 | |||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2310 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| Properties | |||
| C5H8O2 | |||
| Molar mass | 100.117 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 0.975 g/mL[1] | ||
| Melting point | −23 °C (−9 °F; 250 K) | ||
| Boiling point | 140 °C (284 °F; 413 K) | ||
| 16 g/(100 mL) | |||
| −54.88·10−6 cm3/mol | |||
| Hazards | |||
| GHS labelling: | |||
   
| |||
| Danger | |||
| H226, H302, H311, H320, H331, H335, H341, H370, H412 | |||
| P201, P202, P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P273, P280, P281, P301+P312, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P307+P311, P308+P313, P311, P312, P321, P322, P330, P337+P313, P361, P363, P370+P378, P403+P233, P403+P235, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 34 °C (93 °F; 307 K) | ||
| 340 °C (644 °F; 613 K) | |||
| Explosive limits | 2.4–11.6% | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Acetylacetone is an organic compound with the chemical formula CH3−C(=O)−CH2−C(=O)−CH3. It is classified as a 1,3-diketone. It exists in equilibrium with a tautomer CH3−C(=O)−CH=C(−OH)−CH3. The mixture is a colorless liquid. These tautomers interconvert so rapidly under most conditions that they are treated as a single compound in most applications.[2] Acetylacetone is a building block for the synthesis of many coordination complexes as well as heterocyclic compounds.
Properties
[edit]Tautomerism
[edit]| Solvent | Kketo→enol |
|---|---|
| Gas phase | 11.7 |
| Cyclohexane | 42 |
| Toluene | 10 |
| THF | 7.2 |
| CDCl3[3] | 5.7 |
| DMSO | 2 |
| Water | 0.23 |
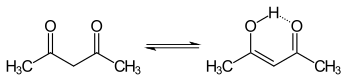
The keto and enol tautomers of acetylacetone coexist in solution. The enol form has C2v symmetry, meaning the hydrogen atom is shared equally between the two oxygen atoms.[4] In the gas phase, the equilibrium constant, Kketo→enol, is 11.7, favoring the enol form. The two tautomeric forms can be distinguished by NMR spectroscopy, IR spectroscopy and other methods.[5][6]
The equilibrium constant tends to be high in nonpolar solvents; when Kketo→enol is equal or greater than 1, the enol form is favoured. The keto form becomes more favourable in polar, hydrogen-bonding solvents, such as water.[7] The enol form is a vinylogous analogue of a carboxylic acid.[citation needed]
Acid–base properties
[edit]| Solvent | T/°C | pKa[8] |
|---|---|---|
| 40% ethanol/water | 30 | 9.8 |
| 70% dioxane/water | 28 | 12.5 |
| 80% DMSO/water | 25 | 10.16 |
| DMSO | 25 | 13.41 |
Acetylacetone is a weak acid. It forms the acetylacetonate anion C5H7O−2 (commonly abbreviated acac−):
- C5H8O2 ⇌ C5H7O−2 + H+

In the acetylacetonate anion, both C-O bonds are equivalent. Both C-C central bonds are equivalent as well, with one hydrogen atom bonded to the central carbon atom (the C3 atom). Those two equivalencies are because there is a resonance between the four bonds in the O-C2-C3-C4-O linkage in the acetylacetonate anion, where the bond order of those four bonds is about 1.5. Both oxygen atoms equally share the negative charge. The acetylacetonate anion is a bidentate ligand.
IUPAC recommended pKa values for this equilibrium in aqueous solution at 25 °C are 8.99 ± 0.04 (I = 0), 8.83 ± 0.02 (I = 0.1 M NaClO4) and 9.00 ± 0.03 (I = 1.0 M NaClO4; I = Ionic strength).[9] Values for mixed solvents are available. Very strong bases, such as organolithium compounds, will deprotonate acetylacetone twice. The resulting dilithium species can then be alkylated at the carbon atom at the position 1.
Preparation
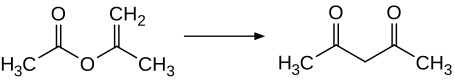
[edit]Acetylacetone is prepared industrially by the thermal rearrangement of isopropenyl acetate.[10]
Laboratory routes to acetylacetone also begin with acetone. Acetone and acetic anhydride ((CH3C(O))2O) upon the addition of boron trifluoride (BF3) catalyst:[11]
- (CH3C(O))2O + CH3C(O)CH3 → CH3C(O)CH2C(O)CH3
A second synthesis involves the base-catalyzed condensation (e.g., by sodium ethoxide CH3CH2O−Na+) of acetone and ethyl acetate, followed by acidification of the sodium acetylacetonate (e.g., by hydrogen chloride HCl):[11]
- CH3CH2O−Na+ + CH3C(O)OCH2CH3 + CH3C(O)CH3 → Na+[CH3C(O)CHC(O−)CH3] + 2 CH3CH2OH
- Na+[CH3C(O)CHC(O−)CH3] + HCl → CH3C(O)CH2C(O)CH3 + NaCl
Because of the ease of these syntheses, many analogues of acetylacetonates are known. Some examples are benzoylacetone, dibenzoylmethane (dbaH)[clarification needed] and tert-butyl analogue 2,2,6,6-tetramethyl-3,5-heptanedione. Trifluoroacetylacetone and hexafluoroacetylacetonate are also used to generate volatile metal complexes.
Reactions
[edit]Condensations
[edit]Acetylacetone is a versatile bifunctional precursor to heterocycles because both keto groups may undergo condensation. For example, condensation with Hydrazine produces pyrazoles while condensation with Urea provides pyrimidines. Condensation with two aryl- or alkylamines gives NacNacs, wherein the oxygen atoms in acetylacetone are replaced by NR (R = aryl, alkyl).
Coordination chemistry
[edit]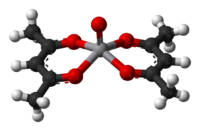
Sodium acetylacetonate, Na(acac), is the precursor to many acetylacetonate complexes. A general method of synthesis is to treat a metal salt with acetylacetone in the presence of a base:[12]
- MBz + z Hacac ⇌ M(acac)z + z BH
Both oxygen atoms bind to the metal to form a six-membered chelate ring. In some cases the chelate effect is so strong that no added base is needed to form the complex.
Biodegradation
[edit]The enzyme acetylacetone dioxygenase cleaves the carbon-carbon bond of acetylacetone, producing acetate and 2-oxopropanal. The enzyme is iron(II)-dependent, but it has been proven to bind to zinc as well. Acetylacetone degradation has been characterized in the bacterium Acinetobacter johnsonii.[13]
References
[edit]- ^ "05581: Acetylacetone". Sigma-Aldrich.
- ^ Thomas M. Harris (2001). "2,4-Pentanedione". e-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rp030. ISBN 0471936235.
- ^ Smith, Kyle T.; Young, Sherri C.; DeBlasio, James W.; Hamann, Christian S. (12 April 2016). "Measuring Structural and Electronic Effects on Keto–Enol Equilibrium in 1,3-Dicarbonyl Compounds". Journal of Chemical Education. 93 (4): 790–794. Bibcode:2016JChEd..93..790S. doi:10.1021/acs.jchemed.5b00170.
- ^ Caminati, W.; Grabow, J.-U. (2006). "The C2v Structure of Enolic Acetylacetone". Journal of the American Chemical Society. 128 (3): 854–857. doi:10.1021/ja055333g. PMID 16417375.
- ^ Manbeck, Kimberly A.; Boaz, Nicholas C.; Bair, Nathaniel C.; Sanders, Allix M. S.; Marsh, Anderson L. (2011). "Substituent Effects on Keto–Enol Equilibria Using NMR Spectroscopy". Journal of Chemical Education. 88 (10): 1444–1445. Bibcode:2011JChEd..88.1444M. doi:10.1021/ed1010932.
- ^ Yoshida, Z.; Ogoshi, H.; Tokumitsu, T. (1970). "Intramolecular hydrogen bond in enol form of 3-substituted-2,4-pentanedione". Tetrahedron. 26 (24): 5691–5697. doi:10.1016/0040-4020(70)80005-9.
- ^ Reichardt, Christian (2003). Solvents and Solvent Effects in Organic Chemistry (3rd ed.). Wiley-VCH. ISBN 3-527-30618-8.
- ^ IUPAC SC-Database Archived 2017-06-19 at the Wayback Machine A comprehensive database of published data on equilibrium constants of metal complexes and ligands
- ^ Stary, J.; Liljenzin, J. O. (1982). "Critical evaluation of equilibrium constants involving acetylacetone and its metal chelates" (PDF). Pure and Applied Chemistry. 54 (12): 2557–2592. doi:10.1351/pac198254122557. S2CID 96848983.
- ^ Siegel, Hardo; Eggersdorfer, Manfred (2002). "Ketones". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a15_077. ISBN 9783527306732.
- ^ a b Denoon, C. E. Jr.; Adkins, Homer; Rainey, James L. (1940). "Acetylacetone". Organic Syntheses. 20: 6. doi:10.15227/orgsyn.020.0006.
- ^ O'Brien, Brian. "Co(tfa)3 & Co(acac)3 handout" (PDF). Gustavus Adolphus College.
- ^ Straganz, G.D.; Glieder, A.; Brecker, L.; Ribbons, D.W.; Steiner, W. (2003). "Acetylacetone-cleaving enzyme Dke1: a novel C–C-bond-cleaving enzyme from Acinetobacter johnsonii". Biochemical Journal. 369 (3): 573–581. doi:10.1042/BJ20021047. PMC 1223103. PMID 12379146.