Diacetyl
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Butane-2,3-dione | |
| Other names
Diacetyl
Biacetyl Dimethyl diketone 2,3-Diketobutane | |
| Identifiers | |
3D model (JSmol)
|
|
| 605398 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.428 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2346 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H6O2 | |
| Molar mass | 86.090 g·mol−1 |
| Appearance | Yellow liquid |
| Density | 0.990 g/mL at 15 °C |
| Melting point | −2 to −4 °C (28 to 25 °F; 271 to 269 K) |
| Boiling point | 88 °C (190 °F; 361 K) |
| 200 g/L (20 °C) | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Harmful, flammable |
| GHS labelling: | |
    
| |
| Danger | |
| H225, H302, H315, H317, H318, H331, H373 | |
| P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P272, P280, P301+P312, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P310, P311, P314, P321, P330, P332+P313, P333+P313, P362, P363, P370+P378, P403+P233, P403+P235, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | External MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
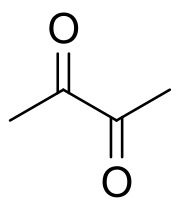
Diacetyl (/daɪjəˈsiːtəl/ dy-yuh-SEE-tuhl; IUPAC systematic name: butanedione or butane-2,3-dione) is an organic compound with the chemical formula (CH3CO)2. It is a yellow liquid with an intensely buttery flavor. It is a vicinal diketone (two C=O groups, side-by-side). Diacetyl occurs naturally in alcoholic beverages and some cheeses and is added as a flavoring to some foods to impart its buttery flavor. Chronic inhalation exposure to diacetyl fumes is a causative agent of the lung disease bronchiolitis obliterans, commonly known as "popcorn lung".
Chemical structure
[edit]A distinctive feature of diacetyl (and other vicinal diketones) is the long C–C bond linking the carbonyl centers. This bond distance is about 1.54 Å, compared to 1.45 Å for the corresponding C–C bond in 1,3-butadiene. The elongation is attributed to repulsion between the polarized carbonyl carbon centers.[2]
Occurrence and biosynthesis
[edit]Diacetyl arises naturally as a byproduct of fermentation. In some fermentative bacteria, it is formed via the thiamine pyrophosphate-mediated condensation of pyruvate and acetyl CoA.[3] Sour (cultured) cream, cultured buttermilk, and cultured butter are produced by inoculating pasteurized cream or milk with a lactic starter culture, churning (agitating) and holding the milk until a desired pH drop (or increase in acidity) is attained. Cultured cream, cultured butter, and cultured buttermilk owe their tart flavour to lactic acid bacteria and their buttery aroma and taste to diacetyl. Malic acid can be converted to lactic acid to make diacetyl.[4][5]
Production
[edit]Diacetyl is produced industrially by dehydrogenation of 2,3-butanediol. Acetoin is an intermediate.[6]
Applications
[edit]In food products
[edit]Diacetyl and acetoin are two compounds that give butter its characteristic taste. Because of this, manufacturers of artificial butter flavoring, margarines or similar oil-based products typically add diacetyl and acetoin (along with beta-carotene for the yellow color) to make the final product butter-flavored, because it would otherwise be relatively tasteless.[7]
Electronic cigarettes
[edit]Diacetyl is used as a flavoring agent in some liquids used in electronic cigarettes.[8] People nearby may be exposed to it in the exhaled aerosol at levels near the limit set for occupational exposure.[9]
In alcoholic beverages
[edit]In some styles of beer (e.g. in many beer styles produced in the United Kingdom, such as stouts, English bitters, and Scottish ales), the presence of diacetyl can be acceptable or desirable at low or, in some cases, moderate levels. In other styles, its presence is considered a flaw or undesirable.[10]
Diacetyl is produced during fermentation as a byproduct of valine synthesis, when yeast produces α-acetolactate, which escapes the cell and is spontaneously decarboxylated into diacetyl. The yeast then absorbs the diacetyl, and reduces the ketone groups to form acetoin and 2,3-butanediol.[citation needed]
Beer sometimes undergoes a "diacetyl rest", in which its temperature is raised slightly for two or three days after fermentation is complete, to allow the yeast to absorb the diacetyl it produced earlier in the fermentation cycle. The makers of some wines, such as chardonnay, deliberately promote the production of diacetyl because of the feel and flavor it imparts.[11] Diacetyl is present in some chardonnays known as "butter bombs", although there is a trend back toward the more traditional French styles.[12]
Concentrations from 0.005 mg/L to 1.7 mg/L were measured in chardonnay wines, and the amount needed for the flavor to be noticed is at least 0.2 mg/L.[13][14]
Use as butter flavoring
[edit]
Butter-flavoring controversy
[edit]Chronic industrial exposure to diacetyl fumes, such as in the microwave popcorn production industry, has been associated with bronchiolitis obliterans, a rare and life-threatening form of non-reversible obstructive lung disease in which the bronchioles (small airway branches) are compressed and narrowed by fibrosis (scar tissue) and/or inflammation.[15][16]
Regulation
[edit]The European Commission has declared diacetyl is legal for use as a flavouring substance in all EU states.[17] As a diketone, diacetyl is included in the EU's flavouring classification Flavouring Group Evaluation 11 (FGE.11). A Scientific Panel of the EU Commission evaluated six flavouring substances (not including diacetyl) from FGE.11 in 2004.[18] As part of this study, the panel reviewed available studies on several other flavourings in FGE.11, including diacetyl. Based on the available data, the panel reiterated the finding that there were no safety concerns for diacetyl's use as a flavouring.[citation needed]
In 2007, the European Food Safety Authority (EFSA), the EU's food safety regulatory body, stated its scientific panel on food additives and flavourings (AFC) was evaluating diacetyl along with other flavourings as part of a larger study.[19]
In 2007, the Flavor and Extract Manufacturers Association recommended reducing diacetyl in butter flavorings.[20] Manufacturers of butter flavored popcorn including Pop Weaver, Trail's End, and ConAgra Foods (maker of Orville Redenbacher's and Act II) began removing diacetyl as an ingredient from their products.[21][22]
A 2010 U.S. OSHA Safety and Health Information Bulletin and companion Worker Alert recommend employers use safety measures to minimize exposure to diacetyl or its substitutes.[23]
In 2016, diacetyl was banned in e-liquids/e-cigarettes in the EU under the EU Tobacco Products Directive.[24]
See also
[edit]- Acetylpropionyl, a similar diketone
- Acetoin
- Bronchiolitis obliterans
References
[edit]- ^ Merck Index (11th ed.). p. 2946.
- ^ Eriks K, Hayden TD, Yang SH, Chan IY (1983). "Crystal and molecular structure of biacetyl (2,3-butanedione), (H3CCO)2, at −12 and −100 °C". J. Am. Chem. Soc. 105 (12): 3940–3942. doi:10.1021/ja00350a032.
- ^ Speckman RA, Collins EB (January 1968). "Diacetyl Biosynthesis in Streptococcus diacetilactis and Leuconostoc citrovorum". Journal of Bacteriology. 95 (1): 174–80. doi:10.1128/JB.95.1.174-180.1968. PMC 251989. PMID 5636815.
- ^ "Scott Labs | Managing Diacetyl Production During MLF". Archived from the original on 2022-07-05. Retrieved 2022-07-11.
- ^ Jay, James M (2000). Modern Food Microbiology. Gaithersburg, Md: Aspen Publishers. pp. 120. ISBN 978-0834216716. OCLC 42692251.
- ^ Siegel H, Eggersdorfer M. "Ketones". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a15_077. ISBN 978-3527306732.
- ^ Pavia DL (2006). Introduction to Organic Laboratory Techniques (4th ed.). Cengage Learning. ISBN 978-0-495-28069-9.
- ^ Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems, National Academies of Sciences (2018). "Chapter 5: Toxicology of E-Cigarette Constituents". In Eaton DL, Kwan LY, Stratton K (eds.). Public Health Consequences of E-Cigarettes. National Academies Press. p. 175. ISBN 9780309468343. PMID 29894118. Archived from the original on 2020-01-07. Retrieved 2019-11-30.
- ^ Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems, National Academies of Sciences (2018). "Chapter 3: E-Cigarette Devices, Uses, and Exposures". In Eaton DL, Kwan LY, Stratton K (eds.). Public Health Consequences of E-Cigarettes. National Academies Press. p. 82. ISBN 9780309468343. PMID 29894118. Archived from the original on 2018-11-18. Retrieved 2018-11-17.
- ^ Janson L (1996). Brew Chem 101. Storey Books. pp. 64–67. ISBN 978-0-88266-940-3.
- ^ "Diacetyl". E. coli Metabolome Database. ECMDB. Archived from the original on 20 October 2013. Retrieved 20 October 2013.
- ^ "Trends in Chardonnay". Sonoma-Cutrer Vineyards. Retrieved December 2, 2015. [dead link]
- ^ Nielsen JC, Richelieu M (February 1999). "Control of flavor development in wine during and after malolactic fermentation by Oenococcus oeni". Applied and Environmental Microbiology. 65 (2): 740–745. Bibcode:1999ApEnM..65..740N. doi:10.1128/AEM.65.2.740-745.1999. PMC 91089. PMID 9925610.
- ^ Martineau B, Henick-Kling T, Acree T (1995). "Reassessment of the Influence of Malolactic Fermentation on the Concentration of Diacetyl in Wines". Am. Soc. Enol. Vitic. 46 (3): 385–388. doi:10.5344/ajev.1995.46.3.385. S2CID 88263667. Archived from the original on 2009-01-07. Retrieved 2009-04-24.
- ^ Merriam-Webster Medical Dictionary > bronchiolitis obliterans Archived 2017-08-01 at the Wayback Machine Retrieved on August, 2010
- ^ Harber P, Saechao K, Boomus C (2006). "Diacetyl-induced lung disease". Toxicol Rev. 25 (4): 261–272. doi:10.2165/00139709-200625040-00006. PMID 17288497. S2CID 42169510.
- ^ "Adopting a register of flavouring substances used in or on foodstuffs drawn up in application of Regulation (EC) No 2232/96 of the European Parliament and of the Council" (PDF). 28 October 1996. Archived from the original (PDF) on November 19, 2007.
- ^ "Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) on a request from the Commission" (PDF). The EFSA Journal. 166: 1–44. 2004.
- ^ "Europe takes 'wait-and-see' stance on diacetyl flavouring". foodnavigator.com. October 31, 2007. Archived from the original on March 8, 2023. Retrieved December 4, 2023.
- ^ "Comments of the Flavor and Extract Manufacturers Association of the United States on New Information on Butter Flavored Microwave Popcorn" (PDF) (press release). FEMA. Archived from the original (PDF) on 2015-10-18. Retrieved 2012-07-25.
- ^ Weaver Popcorn Company. Press Release: Pop Weaver introduces first microwave popcorn with flavoring containing no diacetyl Archived September 28, 2007, at the Wayback Machine
- ^ ConAgra Foods Press Release ConAgra Foods press release announcing removal of added diacetyl Archived 2015-10-18 at the Wayback Machine
- ^ OSHA Recommends Safety Measures to Protect Workers from Diacetyl Exposure Archived 2010-12-21 at the Wayback Machine, EHS Today, December 10, 2010.
- ^ "European Commission – Press Releases – 10 key changes for tobacco products sold in the EU". Archived from the original on 2018-10-20. Retrieved 2018-11-17.
Further reading
[edit]External links
[edit]- Toxicology data
- NIOSH Alert: Preventing Lung Disease and Workers who Use or Make Flavorings
- A Case of Regulatory Failure – Popcorn Workers Lung, from www.defendingscience.org.
- Scientists Urge Secretary of Labor to Protect Workers from Diacetyl, a press release from defendingscience.org. Links to studies on the health effects of diacetyl, and to a variety of related documents including the recent OSHA petition and the scientists' letter of support may be found here.
- Flavoring suspected in illness, Washington Post, May 7, 2007.
- NIOSH International Safety Card for 2,3-butanedione
- National Institute for Occupational Safety and Health – Flavorings-Related Lung Disease
- IFIC – Diacetyl

