Fenchol
Appearance
(Redirected from Β-fenchyl alcohol)
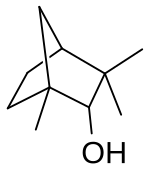 (1R)-endo-(+)-Fenchol
| |
| Names | |
|---|---|
| IUPAC name
(1R,2R,4S)-1,3,3-Trimethyl-2-norbornanol
| |
| Other names
Fenchyl alcohol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.015.127 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H18O | |
| Molar mass | 154.253 g·mol−1 |
| Density | 0.942 g/cm3 |
| Melting point | 39 to 45 °C (102 to 113 °F; 312 to 318 K) |
| Boiling point | 201 °C (394 °F; 474 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Fenchol or 1,3,3-trimethyl-2-norbornanol is a monoterpenoid and an isomer of borneol. It is a colorless or white solid. It occurs widely in nature.
The naturally occurring enantiomere (1R)-endo-(+)-fenchol is used extensively in perfumery. Fenchol gives basil its characteristic scent,[2][3] and comprises 15.9% of the volatile oils of some species of Aster.[4]
It is biosynthesized from geranyl pyrophosphate via isomerization to linalyl pyrophosphate.[5]
Oxidation of fenchol gives fenchone.
References
[edit]- ^ Datasheet at chemexper.com
- ^ "FES - (-)-endo-fenchol synthase, chloroplastic precursor - Ocimum basilicum (Sweet basil) - FES gene & protein". www.uniprot.org.
- ^ Kotan, Recep; Kordali, Saban; Cakir, Ahmet (August 2007). "Screening of antibacterial activities of twenty-one oxygenated monoterpenes". Zeitschrift für Naturforschung C. 62 (7–8): 507–513. doi:10.1515/znc-2007-7-808. PMID 17913064.
- ^ Matasyoh, Josphat C.; Kiplimo, Joyce J.; Karubiu, Nicholas M.; Hailstorks, Tiffany P. (2006). "Chemical composition and antimicrobial activity of essential oil of Tarchonanthus camphoratus". Food Chemistry. 101 (3): 1183–1187. doi:10.1016/j.foodchem.2006.03.021.
- ^ Satterwhite, D. M.; Wheeler, C. J.; Croteau, R. (15 November 1985). "Biosynthesis of monoterpenes. Enantioselectivity in the Enzymatic Cyclization of Linalyl Pyrophosphate to (-)-endo-Fenchol". The Journal of Biological Chemistry. 260 (26): 13901–8. doi:10.1016/S0021-9258(17)38661-1. PMID 4055764.
