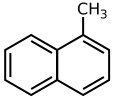
1-Methylnaphthalene
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
1-Methylnaphthalene | |
| Other names
α-methylnaphthalene
| |
| Identifiers | |
3D model (JSmol)
|
|
| 506793 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.788 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 3082 1993 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C11H10 | |
| Molar mass | 142.20 g/mol |
| Appearance | Liquid |
| Density | 1.001 g/mL |
| Melting point | −22 °C (−8 °F; 251 K) |
| Boiling point | 240–243 °C (464–469 °F; 513–516 K) |
| Vapor pressure | 4.91 |
| -102.8·10−6 cm3/mol | |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H302, H304, H411 | |
| P264, P270, P273, P301+P310, P301+P312, P330, P331, P391, P405, P501 | |
| Flash point | 82 °C (180 °F; 355 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
1-Methylnaphthalene is an organic compound with the formula C11H10. It is a colorless liquid. It is isomeric with 2-methylnaphthalene.
Reference fuel
[edit]1-Methylnaphthalene defines the lower (zero) reference point of cetane number, a measure of diesel fuel ignition quality, as it has a long ignition delay (poor ignition qualities). In contrast, cetane, with its short ignition delay, defines the upper reference point at 100.[2] In testing, isocetane (2,2,4,4,6,8,8-heptamethylnonane or HMN) replaced 1-methylnaphthalene as the low cetane number reference fuel in 1962 for reasons of better oxidation stability and ease of use in the reference engine. The scale is unchanged, as isocetane's cetane number is measured at 15, referenced to 1-methylnaphthalene and cetane.[3]
Methylnaphthalene anion
[edit]With alkali metals, 1-methylnaphthalene forms radical anion salts such as sodium 1-methylnaphthalene.
Compared to its structural analog sodium naphthalene, sodium 1-methylnaphthalene is more soluble, which is useful for low-temperature reductions.[4]
See also
[edit]References
[edit]- ^ 1-Methylnaphthalene Archived 2008-05-13 at the Wayback Machine at University of Oxford
- ^ Speight, James G. (2015). Handbook of Petroleum Product Analysis. Hoboken, NJ: Wiley. pp. 158–159. ISBN 978-1-322-95015-0. OCLC 903318141.
- ^ Jääskeläinen, Hannu (2007). Fuel Property Testing: Ignition Quality. DieselNet Technology Guide (Technical report). ECOpoint Inc. Archived from the original on 2019-06-26. Retrieved 2021-02-24.
- ^ Liu, X.; Ellis, J. E. (2004). "Hexacarbonylvanadate(1−) and Hexacarbonylvanadium(0)". Inorg. Synth. 34: 96–103. doi:10.1002/0471653683.ch3. ISBN 0-471-64750-0.
